Dr. Edward Young
 Department of Earth, Planetary, and Space Sciences
Department of Earth, Planetary, and Space Sciences
University of California, Los Angeles
Rare Isotopologues as Tracers of Methane Provenance
Dr. Ed Young has broad research interests, including the origin of primitive meteorites, the nature of oxygen isotope effects in the atmosphere, and the geochemistry of fossil-fuel hydrocarbons. His PRF-supported research is to investigate the formation, mixing, and migration of natural methane (CH4), using the isotopic composition of rare doubly-substituted molecules, which contain both 13Carbon and Deuterium atoms.
Methane in most natural gas reservoirs is biogenic, produced by bacteria, or by thermal cracking of organic material in sedimentary rocks. Methane is also produced by serpenitization, fluid interaction with mafic igneous rocks. Previous research traced the origins of biogenic and abiogenic natural methane by comparing the stable isotope ratios of carbon and hydrogen in methane from different sources. Analysis of the geochemistry of methane by traditional mass spectrometry is complicated because there is overlap of the carbon isotopic ratios of methane produced by thermal cracking, microbial activity, and abiotic sources. Another problem in source identification is that methane in different hydrocarbon reservoirs may have different sources and mixing histories.
Traditional mass spectrometry involved the analysis of stable isotopes in CO2, but the discovery of molecules with isotopes of two heavy atoms led to the development of new techniques to analyze these “clumped isotopes” by mass spectrometry. Young’s current research looks at methane molecules which are chemically identical, but contain different isotopes of Hydrogen and Carbon. He states that “the idea was to use several multiply-substituted molecules (with more than one heavy isotope) as a tracer of how the methane was made.”
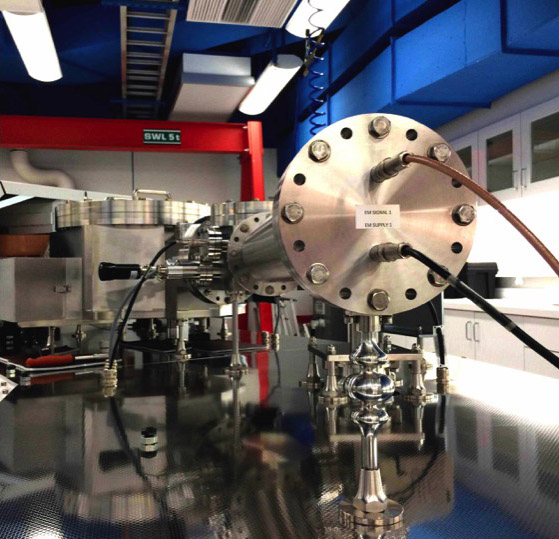
This project began in a quest to build a “bigger and better” mass spectrometer, as the ability of a mass spectrometer to resolve two different gas molecules is m/Δm, where m is atomic mass. Typical mass spectrometers can only resolve about 1/900 of the mass difference between two molecules. The very slight difference in mass between the two doubly-substituted methane “isotopologues” (12CH2D2 is 18.044, and 13CH3D is 18.041) can only be resolved with a very large mass spectrometer. Funding from the Sloan Foundation’s Deep Carbon Observatory project enabled Dr. Young to construct “the world’s largest gas-source isotope ratio mass spectrometer.” The Panorama spectrometer in Young’s UCLA laboratory has mass resolving power of 80K, compared to other mass spectrometers which have “only 25K resolution.”
Young and his students began by mixing samples of different isotopic compositions, synthesizing abiogenic methane samples in the laboratory, and analyzing natural methanes produced by thermogenic alteration of biotic materials. These laboratory-produced samples were compared to field samples of methane gas from different geographic locations. Methane from wells drilled in sedimentary basins, such as samples from the Canadian Shield, showed “huge isotopic effects” from mixing of methane from different sources, as predicted by theory.
Their research showed that abiotic methane samples were deficient in the doubly-deuterated form, and methane geochemistry could be used as a fingerprint for how the molecules were formed, as abiotic samples had different CH3D signatures than biogenic methane. Variations in the concentration of doubly-substituted isotopologues is temperature-dependent, so these data may provide information on the temperature of formation, as thermogenic methane formation occurs at temperatures >140ºC, microbial methanogenesis occurs at 70-90ºC, and methane formed by abiotic serpentinization occurs at temperatures as low as 50ºC.
An unexpected result of this research was the discovery that under the right conditions, methanotrophic bacteria can equilibrate methane to the temperature of their local environment, in contrast to disequilibrium effects predicted from previous studies of stable oxygen and carbon isotopes. Following up on these initial results will require collaboration with microbiologists, to develop theories about how the coenzyme that produced methane can be used to destroy methane in oxidation.
According to Young, “it took us years to raise the funding for the mass spectrometer, and the PRF grant gave us support to explore different avenues simultaneously,” with three students funded at different times on this grant. One student helped set up the Panorama spectrometer, and set up the vacuum extraction system and gas-chromatograph columns for methane gas analysis. Mojhgan Haghnegahdar worked on the theory of atmospheric methane sources and sinks, and her prediction on these rare isotopologues in air was validated by methane samples from Alaskan wetlands. Jeanine Ash studied the biology of methane-producing bacteria, and discovered that methanotrophs can equilibrate methane.
The next steps in Young’s research will be investigating the biology of methanotropic bacteria, to study equilibrium and disequilibrium processes, evaluating the impact of biological oxidation on the mass distribution of methane isotopologues, and assessing whether methane isotopologues may be developed as a tool for petroleum exploration. Based on his interests in astrochemistry, in the future, Young is hoping to analyze methane samples recovered from comets.
Young received a B.A. from the College of Wooster, an M.S. from Vanderbilt University, and a Ph.D. from the University of Southern California, and performed post-doctoral research at the Carnegie Institution of Washington, DC. After several years as a faculty member of Linacre College of the University of Oxford, Dr. Young moved to UCLA, where is he currently a Professor of Geochemistry and Cosmochemistry.
Grant #54848-ND2: Read Young's Annual Report
 Dr. Young's lab
Dr. Young's lab Dr. Young's lab
Dr. Young's lab Dr. Young's lab and students
Dr. Young's lab and students










