Reports: UR754873-UR7: Surface Modification of Polyaniline Films and Nanofibers with Polymer Brushes
Timothy Hanks, Furman University
Introduction: In previous work, we have shown that electrochemically grown thin films of certain intrinsically conducting polymers (ICPs), particularly polypyrrole and polyaniline (PANI), react with thiols to yield surface-modified materials. In this project we are expanding upon this idea in order to grow polymer brushes on the surface of ICP films and nanofibers. Key questions that are being addressed focus on effects of the ICP substrate on the controlled living polymerization reactions used to grow polymer brushes from the surface (known as a 'grafted from' process). These include the atom-transfer radical polymerization (ATRP) and the reversible addition/fragmentation chain Transfer (RAFT) polymerization. Additionally, we are looking to demonstrate the formation polymer brushes on ICP nanorods and particles as these are better suited for eventual practical applications of this technology.
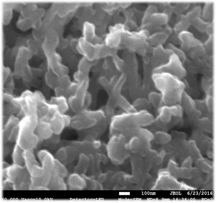
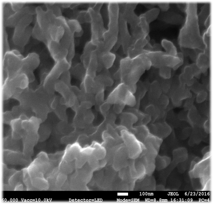
Surface Modification of PANI Nanorods: In an initial study, we have demonstrated that thiol terminated polymers of polyethylene glycol (PEG-SH) can be grafted to the surface of PANI nanorods to form model brush-like surface structures. Nanorods where prepared by a modification of literature procedures and then treated with PEG-SH with molecular weights ranging from 1,000 to 40,000. Figure 1 shows that the morphology of the nanorods is largely unaffected by exposure to the thiols, even under prolonged reflux in water.
Figure 1. FESEM images A (left) and B (right) show PAni nanorods unmodified and modified (25% PEG by weight), respectively.
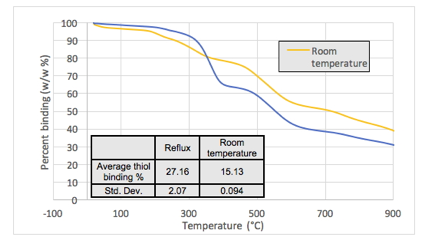
The reaction was found to progress to a point of maximum substitution in about 24 hours at room temperature and 2 hours at reflux using a 1000 MW PEG-SH. Higher temperatures also lead to almost double the amount of thiol covalently bound to the nanorods based on thermal gravimetric analysis (Fig. 2).
Figure 2. Table (inset) illustrates the difference in thiol binding percentage (w/w %) determined from TGA with changing temperature.
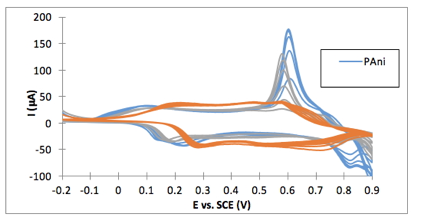
The thiol reaction altered the redox behavior of the nanorods as is evidenced in figure 3, but the material remains electrically conductive. A publication detailing these results is in the final stages of preparation.
Figure 3. CV curve for the polyaniline-coated electrode: (blue) unmodified before reaction with PEG-SH, (orange) after reacting PAni under reflux with PEG-SH, and (grey) after reacting the PAni at room temperature.
ATRP Reactions on PPy Films:
Films of polypyrrole have been electrochemically grown on gold quartz crystal microbalance (ACM)resonators using standard conditions. These were then treated with an ATRP initiator (w-mercaptoundecyl bromoisobutyrate) followed by flowing a degassed aqueous solution of N-(3-Sulfopropyl)-N-(methacryloxyethyl)- N,N-dimethylammonium betaine (SBMA). The monomer solution was flushed across the resonator until the systems stabilized at which time a solution containing both the monomer and a copper catalyst (Cu(I)bipyridine). The ATRP reaction commenced and was monitored by the changes in the resonance frequency and the dampening in the QCM. Simultaneously, and using the same solutions, the ATRP reaction taking place directly on a control resonator where the ATRP initiator was directly bound to the gold resonator surface was also monitored. Under the conditions used, the ATRP reaction on the polypyrrole surface was found to proceed approximately 3 times as fast as that directly on the gold surface. We attribute this to the increased surface area of the polypyrrole surface resulting in a larger number of initiation sites for the polymerization reaction. We have yet to quantify the number of thiols attached to the polymer and cannot directly comment on the relative rates of polymer brush growth, but these results indicate that the ATRP reaction does proceed smoothly on polypyrrole films.