Reports: ND253800-ND2: The Compound-Specific Sulfur Isotopic Signature of Dissolved Organic Matter Sulfurization
Josef P. Werne, PhD, University of Pittsburgh
Organic sulfur (OS) is the second largest pool of reduced sulfur in sediments. Understanding how and why OS forms in oils and source rocks is a key question, and the application of compound-specific S isotope analysis (CSSIA) – an emerging technique that has just recently been developed (Amrani et al. 2009) - to such questions is likely to be informative. Preliminary data applying CSSIA indicate that the sulfur isotope composition (δ34S) of specific organic sulfur compounds (OSC) vary substantially and cover a wide range of values (Raven et al., 2015). Thus, OSCs must derive from multiple pools of inorganic sulfur with δ34S values that vary in time and space. Furthermore, different reaction pathways involving different substrates will impart additional fractionations, leading to a substantial range of δ34S in individual compounds. This study is designed to (1) determine the sulfur isotope fractionation associated with the sulfurization of organic matter and (2) investigate an organic rich sedimentary system to determine whether the sulfur isotope signals expected are preserved in geological samples or overprinted during diagenesis.
Progress to date
Polysulfide analysis
Polysulfides play a significant role in environmentally relevant processes because of their redox reactivity and nucleophilic tendencies, but determining polysulfide speciation in the natural environment is challenging. We are developing methods for the direct measurement of δ34S of each polysulfide species (S22- to S82-) at concentrations present in natural samples using a GC coupled to a multicollector ICP-MS (GC/MC-ICP-MS).
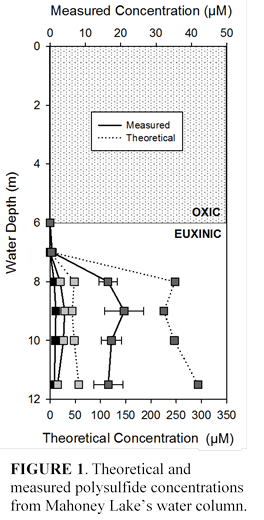
Our refined method currently allows for the complete separation and detection of S22- to S52-, but can be further optimized for lower detection limits and better recoveries. Repeated measurements over a two week period showed that derivatized samples maintain 80% of their original concentration when stored at 2°C. Distributions of dimethylpolysulfanes from the Mahoney Lake, British Columbia water column match theoretical calculations, but the absolute concentrations are offset by an order of magnitude (Figure 1) . The reasons for this offset are most likely explained by (a) inefficient field derivatization, (b) time until extraction and analysis, and/or (c) polysulfides reacting in the water column so their abundance is lower than expected.
δ34S of laboratory-sulfurized compounds
Previous laboratory experiments have suggested that the S isotope fractionation during the formation of OSCs depends on the mechanism of S incorporation and the original functionality of the organic molecule (Amrani et al. 2008). Compound-specific studies of the Cariaco Basin show large variations in the δ34S values of individual OSCs (Raven et al. 2015, Werne et al. 2008). Thus, a key question remains, “What is (are) the sulfur isotope fractionation(s) associated with the sulfurization of organic matter?”
We are analyzing several organic compounds, including carbohydrates (glucose), lipids (5α-Cholestan-3-one), and natural dissolved organic matter sulfurized under conditions representing those in the natural environment. Sulfurized reaction products and associated inorganic sulfur species will be identified and analyzed for their δ34S to determine the isotope fractionation associated with sulfurization.
Compound-specific δ34S of OSC from the Kimmeridge Clay Formation, UK
The Blackstone Band of the Jurassic Kimmeridge Clay Formation (KCF) has an organic carbon content of more than 50%, which has been attributed to the selective preservation of carbohydrates via sulfur incorporation in the euxinic water column (van Dongen et al. 2006), which should be evident in the δ34S values of OSC formed during the process. Comparison of δ34S of KCF OSC to the laboratory-sulfurized compounds will be used to confirm whether the organic enrichment is indeed caused by sulfurization of carbohydrates in the marine water column. Particular attention will be paid to δ34S values of alkylthiophenes – the major reaction products between carbohydrates and inorganic sulfur species (van Dongen et al. 2003) and the most abundant compound class of flash pyrolysates from kerogen of the Blackstone Band (van Dongen et al. 2006).
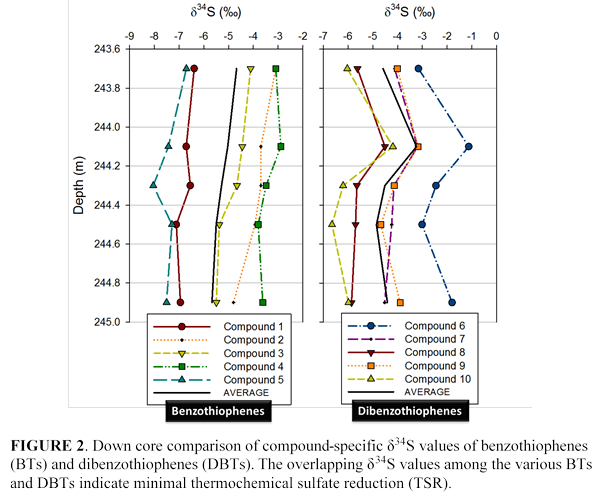
Initial δ34S measurements of lipids, benzothiophenes (BTs) and dibenzothiphenes (DBTs), indicate that the samples have not undergone significant thermochemical sulfate reduction (TSR). BTs are more reactive than DBTs, so there is a greater probability that δ34S values of BTs would be altered, whereas DBTs would preserve their original (kerogen) signal for a longer time (Amrani et al. 2005b). The close correspondence between the δ34S values of BTs (average -5.2‰) and DBTs (average -4.3‰) indicates that TSR has been minimal. This observation confirms that the Blackstone Band is, indeed, thermally immature and shows promise for a compound-specific preservation of an original water column S isotope signal.
Timeline to completion
Sulfurization reactions, identification and quantification of extractable OSC, and inorganic S measurements will be completed Fall 2016. Curie-point pyrolysis-GC will be completed in January 2017 at the University of Manchester with collaborator Dr. Bart Van Dongen. Compound-specific δ34S measurements will be completed in April 2017 at Caltech with collaborator Dr. Alex Sessions.
Impact of research
The research supported by this ACS-PRF grant represents a significant step forward in our understanding of the sulfurization of organic matter in natural systems. The development of improved methods will facilitate the first ever sulfur isotope analyses of individual polysulfides, which is a key factor in understanding this process. The support provided to two graduate students has allowed them to focus full time on their research into organic and stable isotope biogeochemistry, and forms the core of one students PhD dissertation.
References
Amrani A, W. Said-Ahmad, Z. Aizenshtat (2005) Org. Geochem. 36:971-74
Amrani A, Q. Ma, W. Said-Ahmad, Z. Aizenshtat, Y. Tang (2008) Chem. Commun.2008:1356-58
Amrani, A., A. Sessions, J. Adkins (2009) Anal. Chem.81:9027-9034.
Raven, M., A. Sessions, J. Adkins, T. Lyons, J. Werne (2015) Org. Geochem. 80:53-59.
van Dongen B., S. Schouten, J.S. Sinninghe Damsté (2003) Energy Fuels17:1109–18
van Dongen, B., S. Schouten, J.S. Sinninghe Damsté (2006) Org. Geochem. 37:1052-1073.
Werne, J., T. Lyons, D. Hollander, S. Schouten, E. Hopmans, J.S. Sinninghe Damsté (2008) Geochim. Cosmochim. Acta72:3489-3502