Reports: UNI455504-UNI4: Cation-pi Interactions Involving Aromatic Hydrocarbons: A Quantitative Study of Substituent and Solvent Effects
Bright U. Emenike, PhD, State University of New York Old Westbury
Cation-π interactions are applied in many areas of material science including the materials composed of petroleum products. With support from the Petroleum Research Fund (PRF) administered by the American Chemical Society (ACS), my lab's proposed research interests are to measure the strengths of cationic CH-π interactions in solution and to establish quantitative relationships between the interaction energy and the properties of the local environment. In other words, can one accurately predict the interaction energies of the cationic-π interaction in solution? In this first year of the grant-funding period, we have successfully published one peer-reviewed manuscript and have a second manuscript currently under review; both manuscripts directly relate to the objectives of the work described in the grant proposal.
We commenced our studies by developing a molecular torsion balance for measuring very small interaction energies with minimal errors; which resulted in the synthesis of new N-arylimide balances (1 and 2) as test models (Figure 1). Each of the molecular balances adopted a 'folded' and an 'unfolded' conformer (shown in Figure 1) for which the ratio of the conformers in solution provided a quantitative measure of the interaction energy (ΔG) as a function of solvation. In the folded state, the CH donor—which, although not cationic, can be tuned to become polar by the substituents at the aryl's para position—forms an interaction with the naphthalene aromatic ring due to their close proximity. By analyzing the proton NMR spectra of the balances, the energy of the CH-π interaction (ΔG) was measured in various organic solvents, and the data was correlated to a multi-parameter linear solvation energy relationship (LSER) with aims of establishing a mathematical model that could predict the observed experimental values.
Figure 1. Test models (balances 1 and 2) for measuring CH-π interaction
One such LSER equation that was derived from the Kamlet-Taft's solvation model, equation 1, successfully predicted the experimental ΔG values with high accuracy. Additionally, equation 1 also revealed that the hydrogen-bond properties—the hydrogen-bond acceptor (α) and the hydrogen-bond donor (β)—of the local environment are primarily responsible for the balance's conformational preferences. In other words, if the α and β values of a local environment are known, then the environmental effects of solvent on the energy of the CH-π interaction can be predicted. An interesting discovery of equation 1 is that it can also accurately predict the ΔG value for an interaction occurring in the gas phase because all that is needed are the α and β values for conditions mimicking the gas phase. We later corroborated the predicted gas-phase ΔG values through a dispersion-corrected DFT-D (density functional theory) calculation, which resulted in a perfect match. These findings were reported in a paper published in Chemical Science.
ΔG = −0.24 + 0.23α − 0.68β − 0.1π* + 0.09d (1)
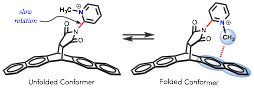
Gratified by the performance of the LSER approach in the test models, we extended the balance design to include a cationic CH-π interaction (balance 3). The rotatable phenyl of balances 1 or 2 was now replaced by a pyridine, which, upon N-methylation, produced the balance 3 with a cationic CH donor. The conformational preferences of balance 3 and closely related analogues were also analyzed by proton NMR spectroscopy and DFT-D calculations. As result of this investigation, we established collaboration with Dr. Matthew Zeller (in the Chemistry department at Purdue University) for solving the X-ray crystal structures of the balance system. In brief, closely related to the conformational studies of balances 1 and 2, we also found that solvent effects (for the cationic balance 3) can be quantitatively rationalized by only the hydrogen-bond properties of the local environment, which contrasts the pervasive idea to over-rely on solvent 'polarity' scales as a means of rationalizing these effects. The findings of this study have culminated in a manuscript that is currently under review.
Figure 2. Model system (balances 3) for measuring cationic CH-π interaction
In the light of these results, we are currently investigating the nature of other cation-π interactions as a function of solvation. We are interested in whether the LSER equations are universal or whether they depend on the nature of the cations in the interaction.
During the first year of this grant, the PRF funds provided for materials, supplies, and undergraduate research stipends. Up to four undergraduate student researchers have been supported through funded positions during the academic year and summer months. These students presented their work at national ACS meetings as well as at local seminars at SUNY Old Westbury. Ultimately, the funding of the PI's and students' salaries in the summer allowed us to spend an extensive amount of time on this project that would not have been possible during the academic year. We have plans to utilize the data from the first year of this grant award to submit an NSF grant proposal in the upcoming year.