Reports: ND254848-ND2: Rare Isotopologues as Tracers of Methane Provenance
Edward Young, PhD, UCLA
We have attained our goal of developing and applying a novel application of rare isotopologues of methane gas for tracing the provenance of CH4 for sites around the globe. Our first paper appeared as the cover article in the International Journal of Mass Spectrometry in 2016 (Figure 1). In that paper we describe the ability to separate the two mass-18 isotopologues of methane, 13CH3D and 12CH2D2. There are two other laboratories measuring the first of these doubly-substituted isotopic species, but our lab is the first to measure the two resolved from one another in natural samples.
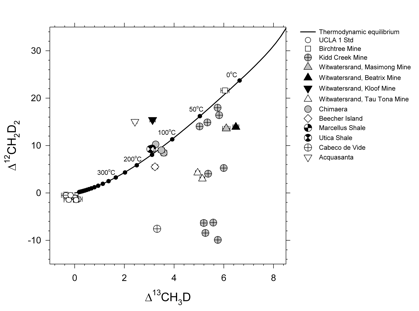
Our first applications have been presented at several scientific meetings (American Geophysical Union, Goldschmidt meeting) and a manuscript with these results was recently submitted for publication. In that work we describe our measurements of methane produced abiotically by the Sabatier reaction (CO2 +4H2 ˆ CH4 + 2H2O) and by decomposition and hydrolysis of methyl-bearing silanes. We also measured samples from axenic cultures of several methanogens. Our natural samples include methane from deep mines in South Africa and the Canadian shield. These samples represent mixtures between abiotic methane and mcirobially-produced and processed methane. We analyzed samples from on-shore ultramafic igneous terranes, including the famous Chimaera seep (flaming rocks of southwestern Turkey) and similar serpentinization products from Portugal and Italy. Samples from the Marcellus and Utica shales of the Appalachian Basin were analyzed as was a sample from the Beecher Island gas field from the Denver Basin, Yuma County, Colorado. A summary of the natural data acquired as part of this work appears in Figure 2.
The ability to resolve 12CH2D2 from 13CH3D provides us with new insights into the origin and evolution of CH4 at many of the sites sampled. The results identify conditions under which either isotopic bond order disequilibrium or equilibrium are expected. Where equilibrium obtains, concordant Δ12CH2D2 and Δ13CH3D temperatures can be used reliably for thermometry. For example, we obtained concordant temperatures of formation for the Marcellus and Utica shales of 140 to 150oC. These robust temperatures are consistent with some models for CH4 generation and inconsistent with some others; the data provide an important arbiter for competing models. We find that concordant temperatures do not always match previous hypotheses based on indirect estimates of temperature of formation nor temperatures derived from CH4/H2 D/H exchange, underscoring the importance of reliable thermometry based on the CH4 molecules themselves. For example, the CH4 from Chimaera yields a robust concordant temperature of formation of 120 to 140oC. This temperature is considerably higher than previous indirect estimates and requires revision to our understanding of the origin of this abiotic gas.
Our work shows that where Δ12CH2D2 and Δ13CH3D values are inconsistent with thermodynamic equilibrium, temperatures of formation derived from these species are suspect or spurious. In such situations, while formation temperatures are unavailable, disequilibrium isotopologue ratios nonetheless provide novel information about the formation mechanism of the gas and the presence or absence of multiple sources or sinks. In particular, disequilibrium isotopologue ratios may provide the means for differentiating between methane produced by abiotic synthesis versus biological processes. Our laboratory experiments show that deficits in 12CH2D2 compared with equilibrium values in CH4 gas made by surface-catalyzed abiotic reactions are so large as to point towards a quantum tunneling origin. The tunneling signature may prove to be an important tracer of abiotic methane formation, especially where it is preserved by dissolution of gas in cool hydrothermal systems.
Our work in the deep mines of the Precambrian shield and with the axenic cultures shows that microbial activity can produce large depletions in both 12CH2D2 and 13CH3D but can also lead to equilibrium Δ12CH2D2 and Δ13CH3D values. Methane gases effusing from fracture waters in the mines have the significant deficits in 12CH2D2 and more modest depletions in 13CH3D that we attribute to abiotic methane formation. These pristine gases are subsequently prone to recycling and isotopic bond re-ordering as microbial communities colonize the fractures from which the gases are issuing over time. The equilibrium clumping signature at host water temperatures in the Birchtree mine sample may be an example of this process going to completion. We are currently analyzing samples of microbially-processed methane from the Baltic Sea that confirm that equilibrium Δ12CH2D2 and Δ13CH3D values are produced at low rates of microbial methanogenesis.
The work described here has provided several graduate students a unique opportunity to work on an important problem with novel instrumentation. Indeed, these students are on the groundfloor of an entirely new way of approaching the problem of using stable isotopes as tracers. The work has afforded all of us opportunities to work with a diverse group of scientist working on problems related to methane formation, and it has brought several groups together who might otherwise not have worked together.
Figure 1. Cover article on the high-resolution gas-source mass spectrometer used for constraining the origins of methane gas.
Figure 2. Δ12CH2D2 and Δ13CH3D for all natural samples in this study. The curve denoting thermodynamic equilibrium is shown for reference. Also shown are replicates of an in-house standard, UCLA-1, as an illustration of external precision.