Reports: UR453212-UR4: Synthesis and Thermophysical Behaviors of Mesothermal Liquid Salts
James H. (Jr.) Davis, PhD, University of South Alabama
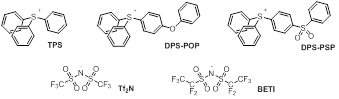
As commonly formulated, ionic liquids (ILs) are generally unstable
at high temperatures for extended periods of time. Rather, while short-term
stability (minutes to perhaps hours) at temperatures in the 250-300+ oC
range is indeed often observed with them, identifying compounds which remain
stable at such temperatures for days, weeks, or longer has been difficult. However, during the first two years of this grant
we showed that ionic liquids pairing the Tf2N anion with select tetraarylphosphonium
(TAP) cations can manifest thermal stabilities far superior to salts of typical
imidazolium-, quaternary ammonium, or tetralkylphosphonium cations likewise
paired with the same or different anions. Indeed, insofar as we were able determine,
several of the species we described were the most thermally stable organic-ion
ionic liquids/molten salts described to that point, and among the most
thermally stable organic materials known, rivalling polymers such as PEEK and
Kapton. We can now report that carefully conceived triarylsulfonium salts (above)
can be added to that list; Better still, the new salts all have Tm
values < 100 oC, with some melting near normal room temperature.
We believe the basis for the enhanced thermal stability of the various per-arylated cation-based salts to be the circumvention of the two most common mechanistic pathways by which alkyl onium cations decompose: the SN2 or E2 removal of an alkyl group from the heteroatom locus of positive charge. Significantly, neither nucleophilic aromatic substitution via an SN2 pathway nor an E2 b-hydrogen abstraction from an arene ring is favourable except in very limited and specific circumstances. Furthermore, a bounty of data is available as to the characteristics of organic molecules which are consistent with a high degree of thermal stability, and these characteristics are the same we are finding in our ongoing work to be compatible with superior thermal stability in ILs.
In addition to identifying triarylsulfonium salts as ionic liquids of high thermal stability, we have carried out an array of experiments to determine how long our perarylated cation salts can endure extreme conditions. We note that for practical applications, being stable at high temperatures for only a few minutes, or only under an inert atmosphere, is unlikely to be of significance for practical applications. Consequently, we examined the thermal stability of a set of tetraarylphosphonium and triarylsulfonium salts of the Tf2N anion in air, at 300 oC, for three months. We were gratified to find that mass loss by these salts was (in all cases) 3% or less of the original material. Further, while some discoloration was present, the salts were spectroscopically indistinguishable from unheated material.
In summary, perarylated salts of P- and S-centered onium cations, when paired with anions of (known) high thermal stability, can confidently be said to constitute the most thermally stable ionic liquids heretofore described. Furthermore, the stability of these species at high temperatures, in air, for long periods of time augers well for their utilization as fluids capable of withstanding high-stress industrial and commercial conditions. Finally, we note that thanks to the results generated as a consequence of ACS-PRF funding, we have successfully applied for and received an NSF grant to continue work on these novel materials.











