Reports: ND154452-ND1: Metal-Free Functionalization of Aromatic Rings - Remarkably Mild Carbon--Heteroatom and Carbon-Carbon Bond Formations, and Dehydrogenative Cross-Coupling Reactions
William Chain, PhD, University of Delaware

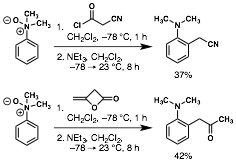
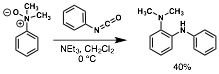
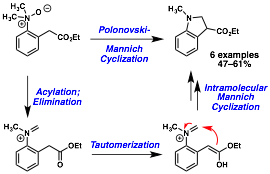
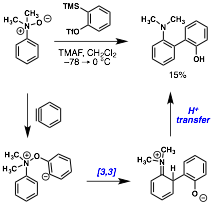
William Chain, PhD, University of Delaware





Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society