Reports: DNI752701-DNI7: Square Arrays of Cylinders in Thin Films of Symmetric ABC Triblock Copolymers
Chuanbing Tang, PhD, University of South Carolina
(1) Symmetric PEO-b-PS-b-PI ABC Triblock Copolymers
We first chose a symmetric PEO-b-PS-b-PI ABC triblock copolymer. The Flory−Huggins χ parameters between PEO, PS, and PI has the scaling relationship χAB ≈ χBC ≪ χAC, which satisfies the conditions for superlattice structures, suggesting that two alternating domains of PEO and PI could be formed in a matrix of PS.
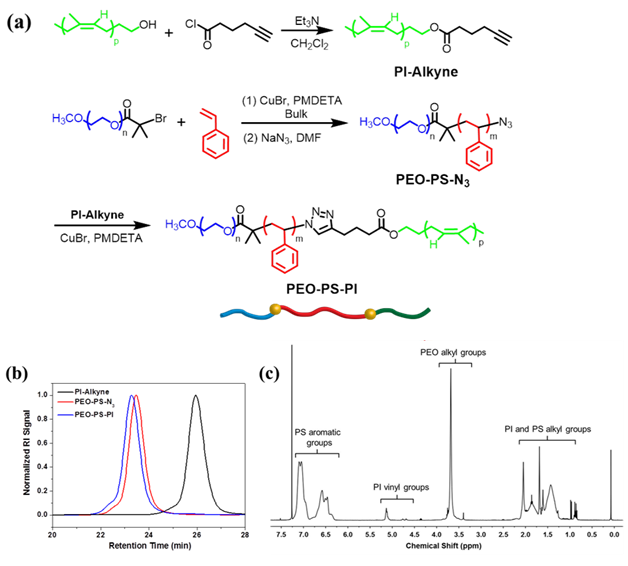
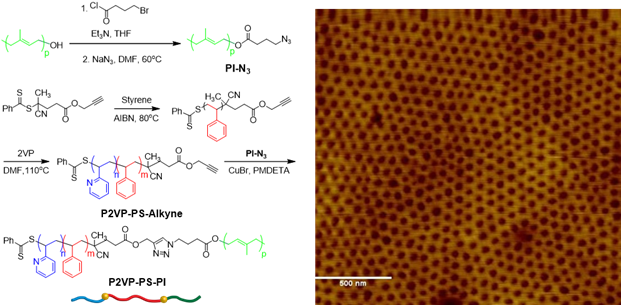
Triblock copolymers (PEO-PS-PI; OSI) containing nearly equal volume fractions of PEO and PI blocks (fPEO ≈ fPI) were synthesized by ATRP and click chemistry, as outlined in Figure 1a. GPC traces demonstrated clean triblock copolymers with low dispersity (Figure 1b). 1H NMR shows all relative peaks for each block in OSI (Figure 1c). Table 1 lists sample information.
Table 1. Characteristic of OSI triblock copolymers.
Figure 1. (a) Synthesis of OSI; (b) GPC traces of OSI; (c) 1H NMR Spectrum.
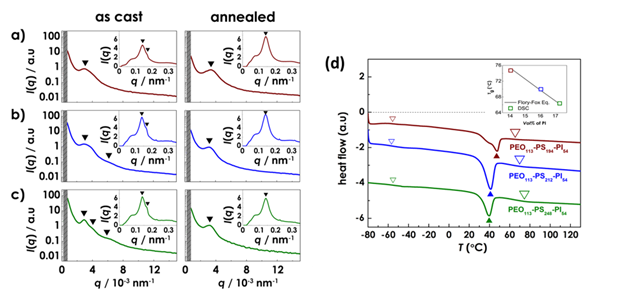
SAXS measurements showed the presence of distinctive scattering peaks in all systems suggesting microdomain formation (Figure 2a-c). Higher order peaks in OSI3 indicate the presence of periodic microdomain morphology. The three peaks in the OSI3 intensity profile correspond to q/q* values of 1, √2, √4 are consistent with a BCC structure. The WAXS inset in Figure 2a-c reveals a distinctive peak for all systems that is similar to reported values for the monoclinic crystal polymorph of PEO.
Figure 2. . SAXS profiles of OSI1 (panel a), OSI2 (panel b), and OSI3 (panel c). WAXS profiles shown in the insets. (d) DSC curves of OSI1, OSI2 and OSI3.
DSC heat flow (Figure 2d) for all of the OSI systems revealed a glass transition and melting point at Tg = -55 °C and Tm = 47~40°C, which is consistent with PEO. There is no indication of a glass transition for PS or PI, but a shallow glass transition at T = 65~76 °C indicative of the softening of a mixed PS/PI domain. This suggests microphase separated structure of PEO in matrix of PS/PI. This is a quite surprising result, different from our initial hypothesis.
Figure 3. (a-c) AFM height images of OSI 3 films, (a) without LiCl; (b) [O]:[Li+] = 16:1; (c) [O]:[Li+] = 8:1. (d-f) GISAXS in plane line cuts for OSI at αi = 0.19o, (d) without LiCl; (e) [O]:[Li+] = 16:1; (f) [O]:[Li+] = 8:1.
Thin film ordering was investigated under a high humidity solvent annealing with and without the incorporation of lithium salt. AFM and GISAXS measurements determined the ordering in the films. Figure 3a-c show ordered hexagonal packed PEO domains with increasing salt concentration. GISAXS measurements were consistent with AFM, showing higher order peaks that are indicative of in-plane hexagonal symmetry (Figure 3d-f).
(2) Symmetric P2VP-b-PS-b-PI ABC Triblock Copolymers
The second triblock composition is P2VP-b-PS-b-PI (ISP), which was prepared by a combination of RAFT and click reaction as shown in Figure 4. AFM showed the formation of hexagonal arrays. We will further characterize these films using TEM, SAXS, and GISAXS to determine the morphology in bulk and thin films. Different compositions of the ISP triblock will be synthesized to determine the role of volume fraction of each block with the morphology of the system.
Figure 4: Synthesis and thin film AFM image of P2VP-PS-PI triblock copolymer.
(3) Solvent Annealing Processes for PS-b-PEO Block Copolymer
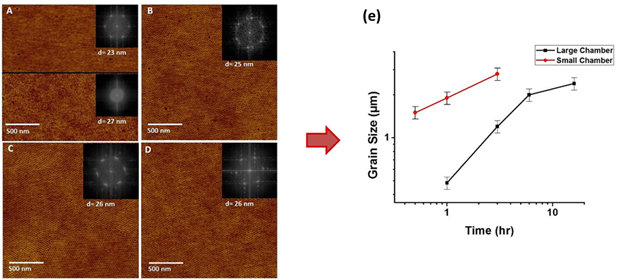
We addressed some challenges in humidity-controlled solvent annealing of PS-b-PEO: (1) reduction in annealing time; (2) utilization of industry-benign solvents. Figure 5e illustrates that annealing time is reduced nearly an order of magnitude by simply using the smaller chamber to achieve lateral ordering when toluene is the solvent. Two industrially benign solvents, methyl ethyl ketone (MEK) and propylene glycol monomethyl ether acetate (PGMEA), were implemented and achieved ordered surface patterns. For toluene, the rate of block copolymer ordering is controlled by the time required to saturate the vapor phase. The kinetics with MEK and PGMEA are more complicated: these solvents reduce the incompatibility between PS and PEO, requiring longer annealing times.
Figure 5. AFM height images of thin films PEO-b-PS under high humidity solvent annealing in toluene in the small chamber: (A =0.25 hr; B = 0.5 hr; C = 1 hr; D = 3 hr. (E) = Lateral grain size in PEO-b-PS thin films as a function of time.
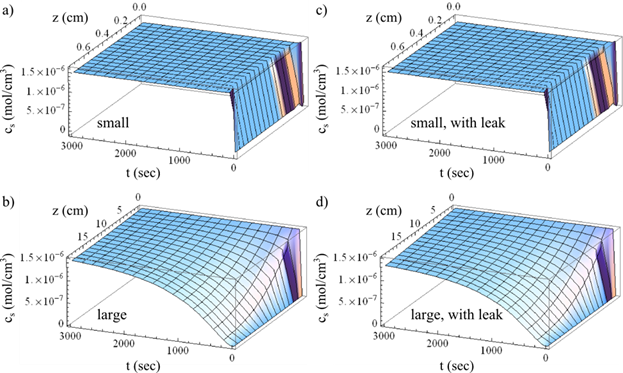
Enhanced ordering kinetics is modeled using numerical calculations from numerous variables from solvent annealing under toluene. As shown in Figure 6, the small chamber can quickly saturated with vapor. However, the large chamber requires nearly an hour to reach the same steady state.
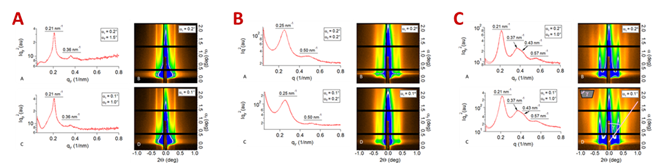
GISAXS scattering profiles (Figure 7a-c) indicate the formation of hexagonal packed domains. Broad “streaks” along the αf axis at all incident angles were observed, suggesting the surface structure dominates the scattering. Toluene and PGMEA indicate hexagonal packing, but MEK scattering profiles are consistent with parallel domains. Therefore the domains at the air interface are parallel cylinders perforated at the top of the film.
Figure 6: Toluene concentration in the vapor phase cs as a function of t and z for a) small chamber; b) large chamber; c) leaky small chamber; d) leaky large chamber.
Figure 7: GISAXS measurement of films by solvent annealing in: toluene (A); MEK (B); PGMEA (C) at (αc ≈ 0.16º).
Research Impact:
(1) The OSI triblock copolymer elaborates on assumptions from earlier work by Bates and Morse groups pertaining to PS/PI mixing. Annealing time is reduced by an order of magnitude by decreasing the volume of the annealing chamber; industry benign solvents produce lateral order at the air interface.
(2) The PI (Chuanbing Tang) was promoted to associate professor and college of arts and sciences distinguished professor. Jeffery Hayat, a 5th-year graduate student is expected to graduate at the end of 2015. Two postdocs were partially supported under this project.