Reports: UR1053827-UR10: Bringing Undergraduates Into Lab-Based Research and Development (BUILD) for Effective Shale Gas Storage Using Macroporous Adsorption Complexes (MACs)
Jingbo Liu, PhD, Texas A&M University-Kingsville
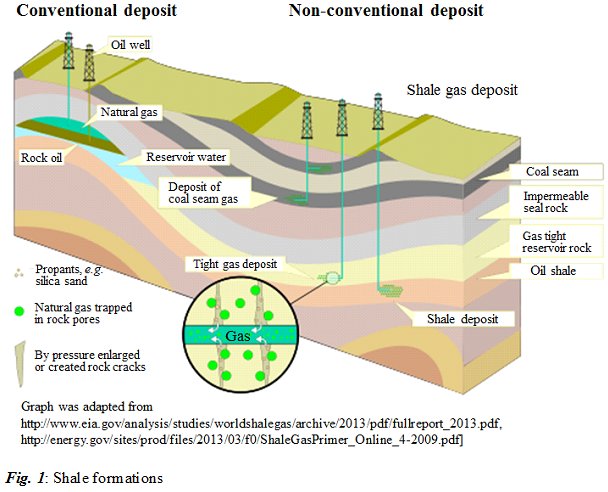
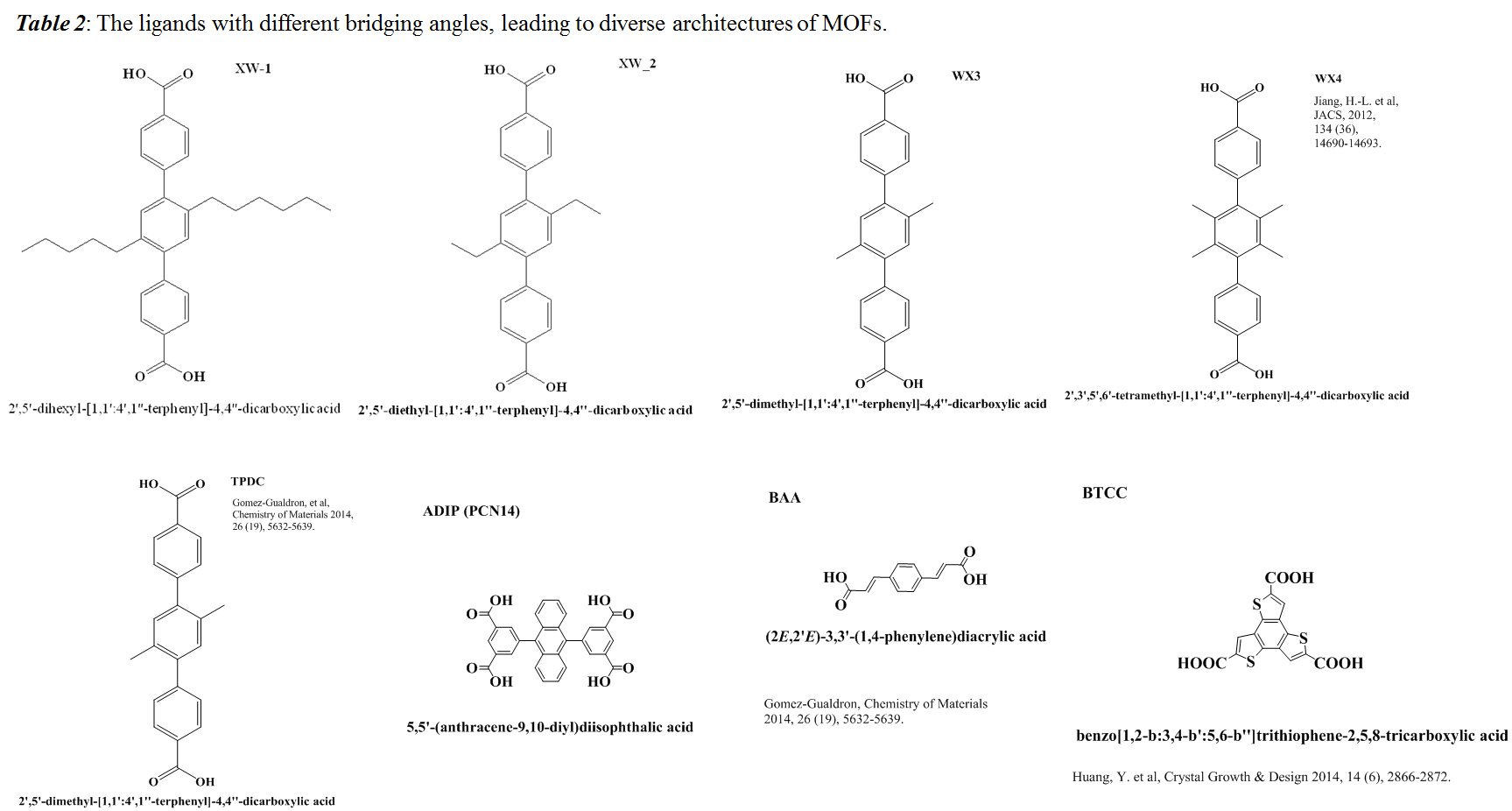
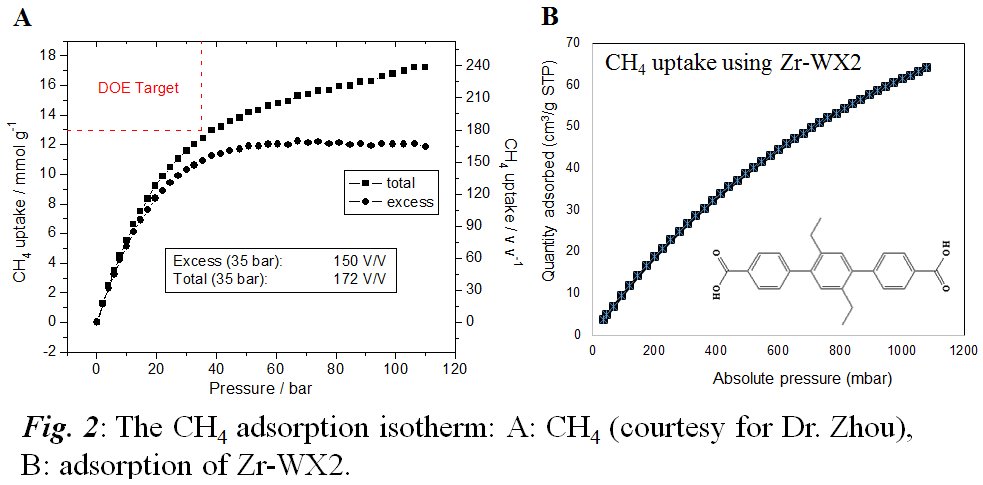
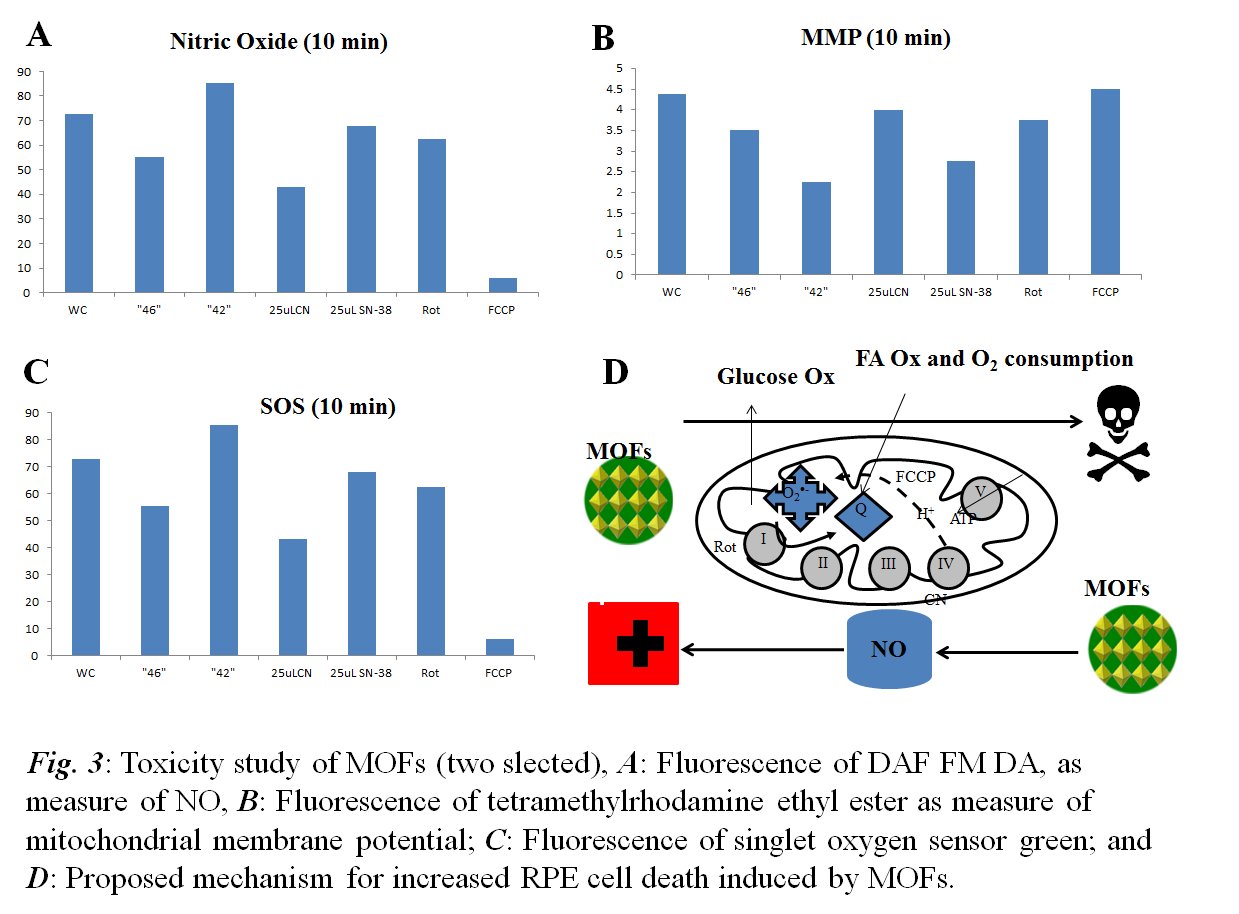
Jingbo Liu, PhD, Texas A&M University-Kingsville




Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society