Reports: UNI653818-UNI6: Extending the Applications of SALDI-MS for Asphaltene Analysis Using Transition Metal Oxide Nanopowders
Karen Virginia Sinclair Molek, PhD, University of West Florida
The purpose of this research is to extend solvent-free investigations using transition metal oxide (TMO) nanoparticles as surfaces for the “soft” ionization/desorption of asphaltenes and petroleum products using SALDI-MS. This research expands current state-of-the-art analysis of these virtually insoluble high molecular weight asphaltenes. Accessibility of instrumentation capable of asphaltene detection and determination of petroleum composition and properties via mass spectrometry are invaluable, particularly during times of high demand such as the 2010 BP Horizon Oil Spill. The current asphaltene analysis research extends previous “capture and removal” of asphaltene studides, via TMO nanoparticles, to investigate utility of TMO nanoparticles as surfaces in SALDI-MS.
RESEARCH PROGRESS:
Three TMO nanoparticles (NiO, Co3O4 and Fe3O4) were tested for asphaltene adsorption efficiency and subsequently analyzed using SALDI-MS. Asphaltenes were donated by Dr. Alan Marshall’s group at the National High Magnetic Field Lab and were precipitated by Dr. Amy McKenna a co-workers using IP 143/90.
A. Adsorption of Asphaltenes
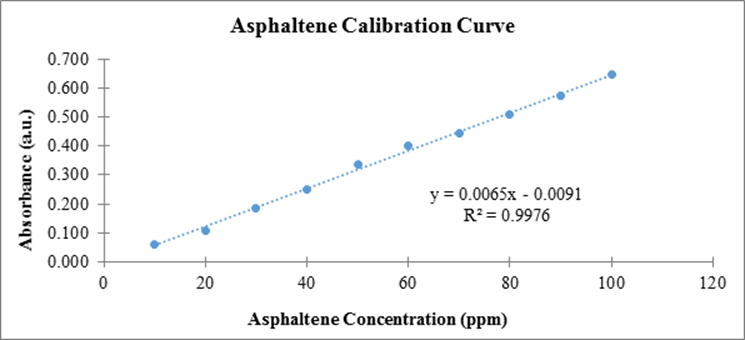
UV-Vis absorption measurements were used to quantify asphaltene adsorption. Absorbance data measured at 375 nm of asphaltene solutions, 10 – 100 ppm, were used to generate a calibration curve, Figure 1.
Figure 1. Experimentally determined calibration curve using asphaltene solutions.
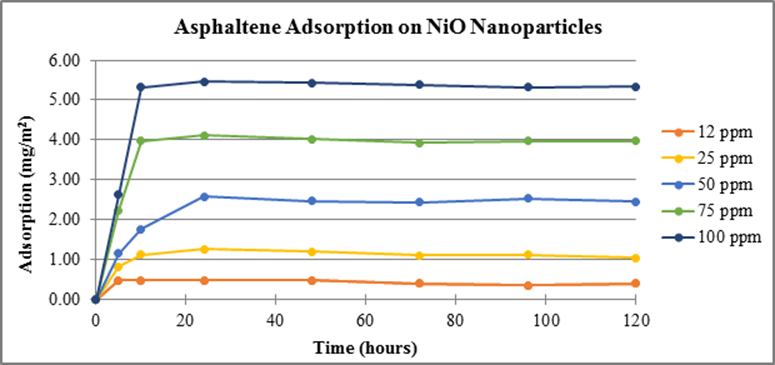
A consistent mass of each TMO nanoparticle was added to the asphaltene stock solutions. Control experiments using NiO, 50 nm diameter, nanoparticles ensured our method and data correlated correctly with literature measurements. The calibration curve, Figure 1, was used in combination with measured absorbance data to determine the concentration of asphaltenes adsorbed after introduction of TMO nanoparticles, as a function of time. Time zero is the measurement taken immediately after TMO nanoparticles are introduced. Adsorption results using NiO nanoparticles, shown in Figure 2, were consistent with literature.
Figure 2. Adsorption of asphaltenes on NiO nanoparticles measured at 375 nm as a function of time.
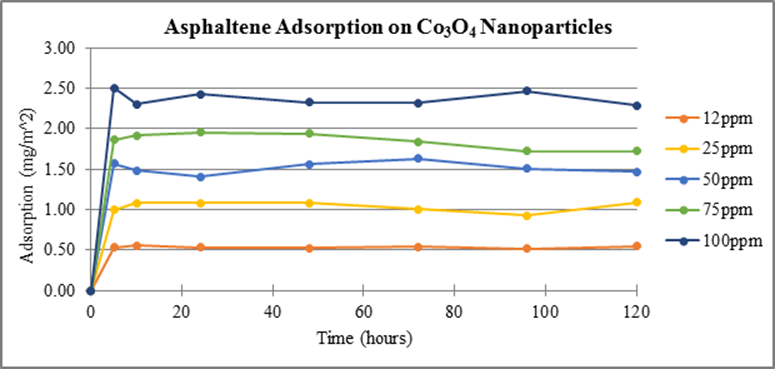
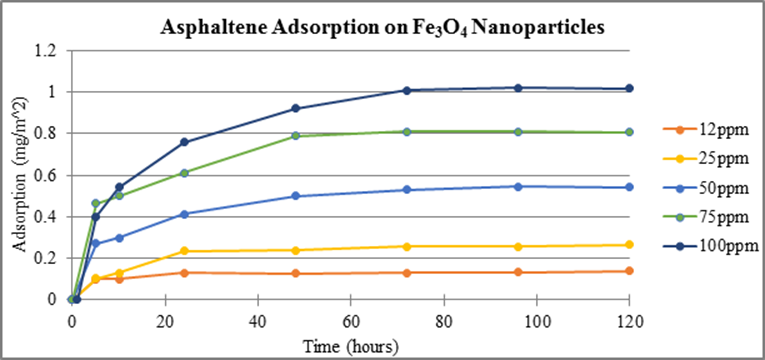
Consistent experimental methods were used to measure adsorption of asphaltenes on 50 nm Co3O4 and 10 nm Fe3O4 nanoparticles, Figures 3 and 4, respectively. Experimental results show adsorption occurs on both cobalt and iron TMO nanoparticles with a larger concentration adsorbing on Co3O4 per unit area. Previous data showed the TMO’s adsorbed asphaltenes according to the following trend: NiO > Co3O4 > Fe3O4. This same trend is shown in our data.
Figure 3. Adsorption of asphaltenes on Co3O4 nanoparticles measured at 375 nm as a function of time.
Figure 4. Adsorption of asphaltenes on Fe3O4 nanoparticles measured at 375 nm as a function of time.
These data show that nickel and cobalt reach equilibrium by the 24 hour time sampling whereas iron reaches equilibrium by the 72 hour mark. New experiments have begun using all three nanoparticles with the goal of attaining additional measurements between 0-24 hours. These data will provide information necessary to determine a more accurate time period at which equilibrium occurs for each TMO.
B. SALDI-MS of Adsorbed Asphaltenes using TMO surfaces
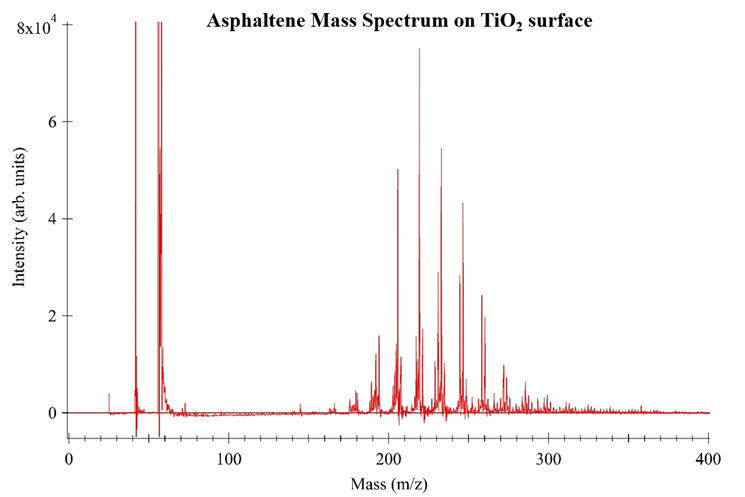
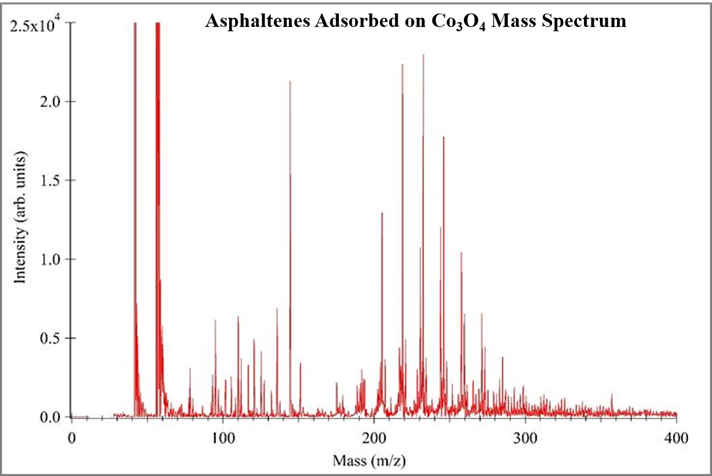
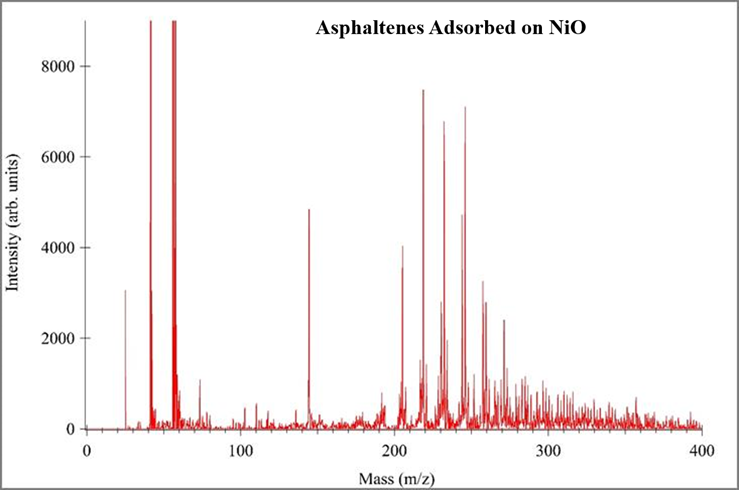
Proof-of-concept experiments demonstrate the utility of TMO nanoparticle surfaces in SALDI-MS analysis of asphaltenes, Figure 5-7. In each experiment 100 ppm asphaltene solutions were used, a consistent mass of TMO nanoparticles were introduced into the asphaltene solution, the solution was equilibrated and the adsorbed asphaltenes were subsequently analyzed via mass spectrometry. A consistent volume and concentration of TiO2 was used as the surface for each analysis. These data show “cleaner” mass spectra as compared to traditional MALDI-MS.
Figure 5. SALDI Mass Spectrum of 100 ppm Asphaltene solution on TiO2 surface.
Figure 6. SALDI Mass Spectrum of 100 ppm Asphaltene solution adsorbed on Co3O4 nanoparticles with TiO2 surface.
Figure 7. SALDI Mass Spectrum of 100 ppm Asphaltene solution adsorbed on NiO nanoparticles with TiO2 surface.
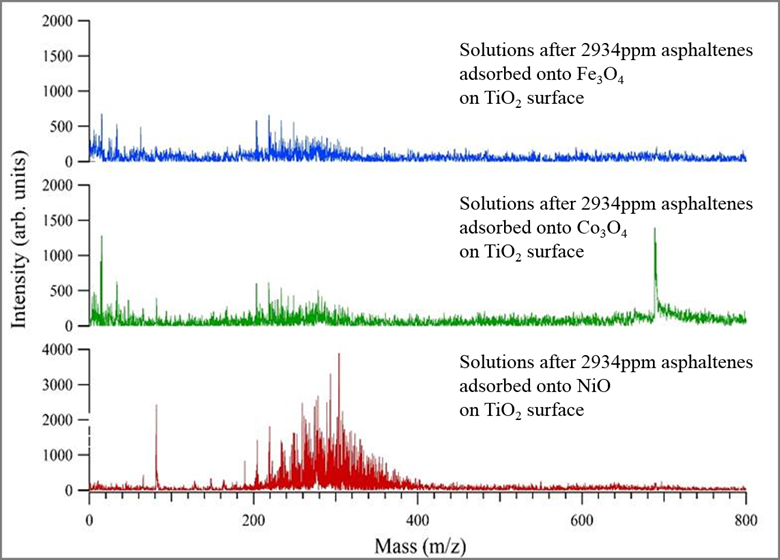
These mass spectra prove TiO2 can be used a surface to analyze asphaltenes adsorbed onto other TMO nanoparticles using SALD-MS. The data also suggests the monomer asphaltene mass is ~230 amu. Literature shows solutions of asphaltenes with a concentration greater than 100 ppm promote aggregation and possibly self-association. The self-association of asphaltenes in high concentration solutions result in higher mass measurements. Figure 8 shows the results of three ~3000 ppm asphaltene solutions prepared the same as samples in Figures 5-7. These spectra provide evidence for self-association showing the shift in mass range. Unlike Figure 5-7, Figure 8 data points toward an improved adsorption capacity of NiO resulting in improved asphaltene detection via SALDI-MS.
Figure 8. Mass spectra of ~3000 ppm asphaltene solutions adsorbed onto respective TMO nanoparticles and analyzed using SALDI-MS on a TiO2 nanoparticle surface.
FUTURE EXPERIMENTS:
Thermogravimetric analysis experiments are underway to determine the effect of adsorption on asphaltene catalytic activity for all solution concentrations. SALDI-MS experiments are underway to determine if each TMO can act as its own surface without the use of TiO2 by analyzing each concentration of asphaltene solution for all three TMO’s with and without the TiO2 surface. The samples will also be run at the National High Magnetic Field Lab on the ICR-FT-MS for more accurate mass spectral analysis.
STUDENT PARTICIPATION, PUBLICATIONS AND PRESENTATIONS:
A total of eleven undergraduate chemistry majors have worked on this project, each for 3 – 5 semesters. The first three students have graduated and are graduate school working towards a PhD in Chemistry. Three of the undergraduate students were supported via grant funds and three additional students were supported by the UWF Summer Undergraduate Research Program. All eleven undergraduate students have presented a eleven poster presentations at the following conferences/symposia: two at the Spring 2014 National ACS meeting (one was invited to participate in Sci-Mix), two at the Spring 2015 National ACS meeting, three at the Annual Biomedical Conference for Minority Students (ABRCMS), and five at the UWF Student Scholars Symposium (one Best STEM Poster and one Best Chemistry Poster). Grant travel funding was used to support the four ACS and three ABRCMS presentations. A manuscript is in preparation detailing the SALD-MS results. The PI will present the results at the Spring 2016 National ACS meeting and three abstracts will be submitted for students to present research posters at the Spring 2016 National ACS meeting.