Reports: ND353315-ND3: Rational Synthesis of Iridium(III)-Alkane Complexes
Thomas Gerald Gray, PhD, Case Western Reserve University
Rational Syntheses of Iridium(III)-Alkane Complexes
The focus of research in the Gray laboratory continues to be metal–carbon bond formation. In a new direction, the Gray research group is extending the chemistry to include metal–alkane sigma-complexes. We are especially interested in exploiting the substitutional inertness of iridium(III) and platinum(II) to stabilize metal-alkane sigma-complexes, while recognizing that alkanes are among the most labile ligands.
Energy resources of the future will expel lesser quantities of CO2 than those of the past. Of immediate importance are alkanes, which have the lowest carbon footprint of all hydrocarbons. Continued experimentation is needed for metal complexes to achieve their potential in the production and refining of natural gas and petroleum. Alkanes are the major components of these fossil fuels. Stable alkane complexes that have been characterized in solution are scarce, and the few established examples have drawn careful scrutiny.
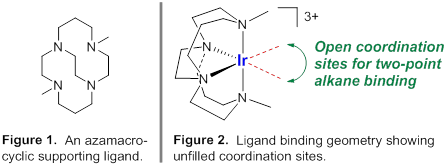
Thermally stable alkane sigma-complexes are sought for iridium(III) and more recently for platinum(II). These metal ions are chosen for their substitutional inertness. Complexes are targeted that bind alkanes through two coordination sites, as bidentate pseudo-ligands.
A library of iridium(III) complexes is being synthesized with neutral, tetradentate supporting ligands. The bound alkane occupies two sites in an octahedral coordination sphere. One supporting ligand is the cross-bridged cyclam appearing in Figures 1 and 2. The Gray research group is developing transformations of iridium(III)-halide precursors that afford sigma-alkane complexes; progress is ongoing. Current work emphasizes the synthesis of halide complexes, de-halogenation in the presence of excess alkane (norbornane), and characterization of new complexes at low temperatures.
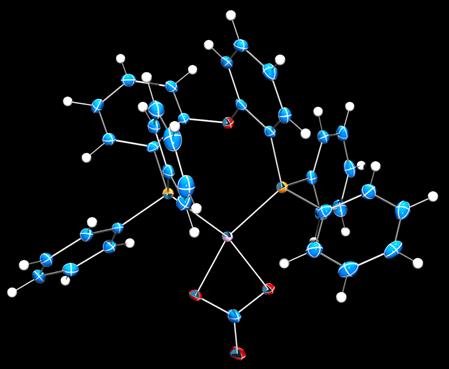
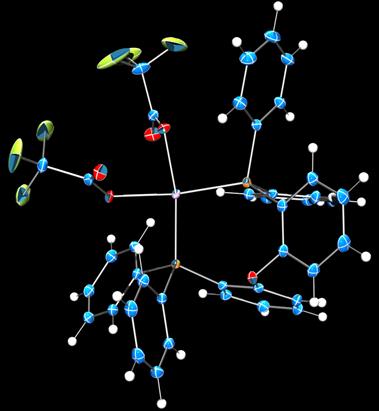
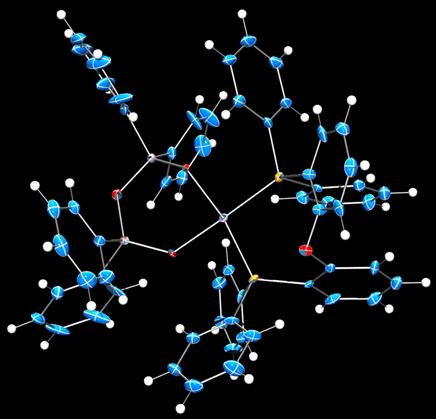
During the past year, we have expanded the project to include sigma-alkane complexes of platinum(II). Four-coordinate platinum(II) is square planar, d8, and substitutionally inert. A variety of platinum(II) precursors has been screened for alkane complex formation, with various reactions to remove the capping non-phosphine ligand. In all cases, the supporting phosphine is electrically neutral to maximize the binding avidity of the metal site. Figures 3–5 depicts crystal structures of representative examples.
Figure 3. Crystal structure of a (DPEphos)platinum(II) carbonato complex (50% probability, 100 K). Legend: Blue, carbon; red, oxygen; orange, phosphorus; violet, platinum; white, hydrogen.
Figure 4. Crystal structure of a (DPEphos)platinum(II) trifluoroacetato complex (50% probability, 100 K). Legend: Blue, carbon; red, oxygen; green, fluorine; orange, phosphorus; violet, platinum; white, hydrogen.
Figure 5. Crystal structure of a (DPEphos)platinum(II) tetraphenyldisiloxanedionato complex (50% probability, 100 K). Legend: Blue, carbon; red, oxygen; grey, silicon; orange, phosphorus; violet, platinum; white, hydrogen.
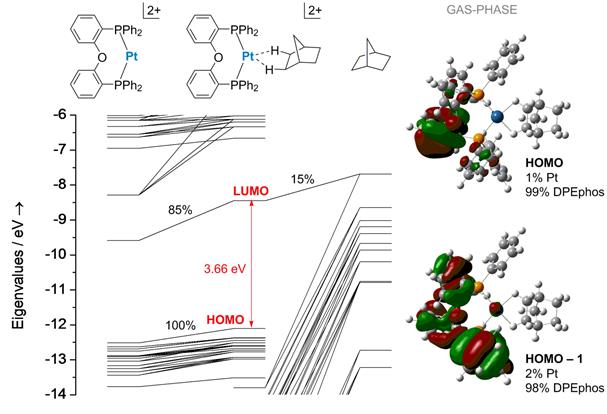
Density-functional theory studies indicate that (diphosphine)platinum(II) fragments are competent to bind cyclic alkanes. An example is the (DPEphos)Pt(II) moiety appearing in Figures 3-6. The norbornane binding enthalpy of this complex is calculated to be 15 kcal/mol (PBE0/TZVP/SDD; corrected for basis set superposition error). Figure 6 depicts a frontier orbital energy diagram of (DPEphos)Pt(norbornane)2+. In the optimized structure, norbornane latches in a pseudo-bidentate structure. That is, the alkane occupies two coordination sites. Binding through two sites, instead of one, impedes alkane loss. The highest energy occupied orbitals reside on the arylphosphine ligand. No orbital within 1.6 eV of the HOMO derives more than 5% of its electron density from norbornane.
Figure 6. Frontier Kohn-Sham orbital energy level of a representative sigma-alkane complex. Depictions of orbitals and compositions in terms of fragment orbital densities appear at right.
Impact of the Research.
This work is enabling the design of molecular alkane complexes that are kinetically stable near room temperature. Although metal-alkane sigma-complexes can be made rationally, their lability usually defeats characterization except at low temperatures. This PRF-supported research seeks robust metal-alkane species. The choices of iridium(III) and platinum(II) are deliberate. Both metals are substitutionally inert, and hence “sticky.” Reliable access to thermally stable alkane complexes will foster the development of more efficient methods to refine crude oil and natural gas.
ACS PRF ND support has permitted the Gray laboratory to begin a major new project in metal-alkane complexes. Essential preliminary data are being gathered for eventual federal funding. Two graduate research assistants have been supported with these funds. Students are trained in Schlenk and inert-atmosphere techniques, and this training was made possible by PRF support.