Reports: UR452099-UR4: Mechanisms and Dynamics of Carbene Additions to Anti-Bredt Olefins
Dina C. Merrer, PhD, Barnard College
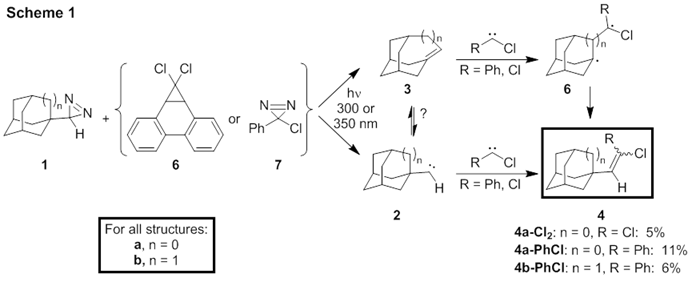
The Platz group previously found that noradamantyldiazirine (1a) reacts through its excited state to give adamantene, 3a, bypassing noradamantylcarbene (2a) (Scheme 1).2 They also found that a greater barrier for the conversion of adamantylcarbene 2b to 3b accounts for a lifetime of 2b long enough to be measured by nanosecond laser flash photolysis (LFP).2
We thus synthesized diazirines 12 and co-photolyzed each with phenylchlorodiazirine (7) at 350 nm at room temperature in pentane; 1a was also co-photolyzed with the phenanthride precursor of CCl2, 6, at 300 nm. Isolation, purification, and characterization of the products led to 11% of 4a-PhCl with slight diastereoselectivity and 6% of 4b-PhCl with no diastereoselectivity, and 5% of 4a-Cl2.
The formation of 4 surprised us. We examined its origins experimentally via NMR and nanosecond LFP. 1H NMR showed the reaction of 1a + 7 to be independent of solvent, and the rate of consumption of 1a correlated with the rate of formation of ethylene 4a-PhCl. In collaboration with Prof. Dasan Thamattoor (Colby College), LFP showed that PhCCl + 3a to be independent of [3a]. The reaction of PhCCl + 3b produced ethylene products 4b-PhCl with k = 9.6 x 105 M-1s-1.
A computational study of these two systems was carried out in collaboration with the Tantillo group (UC Davis). The calculations (UB3LYP/6-31+G(d,p) and UM062X/6-31+G(d,p)//UB3LYP/6-31+G(d,p)) illuminated the likely mechanisms, showing the formation of exocyclic ethylenes 4 to proceed either via (a) on the singlet surface via stepwise addition of PhCCl to bridgehead alkenes 3, producing an intermediate singlet diradical in each case, or (b) via addition of PhCCl to the diazo analogues of diazirines 1. Preliminary direct dynamics calculations on 3a + PhCCl showed a high degree of recrossing (68%), indicative of a flat transition state surface. Overall, 9% of the total trajectories formed 4a-PhCl product, each proceeding via the computed singlet diradical, 6a-PhCl.
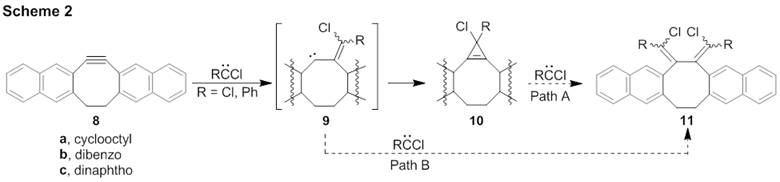
Although the following does not involve anti-Bredt olefins, we have begun to investigate the addition of phenylchlorocarbene to cyclooctynes (Scheme 2). We previously found the addition of RCCl (R = Ph,Cl) to cyclooctyne, 8a, to be barrierless, leading to the corresponding cyclopropenyl product 10a as the global energy minimum.3 While it was possible that the reaction path proceeded via vinylcarbene 9a, the probability of this reaction route was predicted to be low because 9a sits in a shallow energy well. In collaboration with the Houk group (UCLA), we have conducted direct dynamics calculations on CCl2 + 8a to investigate the efficacy of 9a. Without a transition state from which to initiate the trajectories, we initiated a small sample of trajectories (22) from selected starting geometries of CCl2 + 8a. These trajectories either proceeded to cyclopropene 10a or recrossed to starting materials, thus implying that RCCl + 8a is not influenced by reaction dynamics.
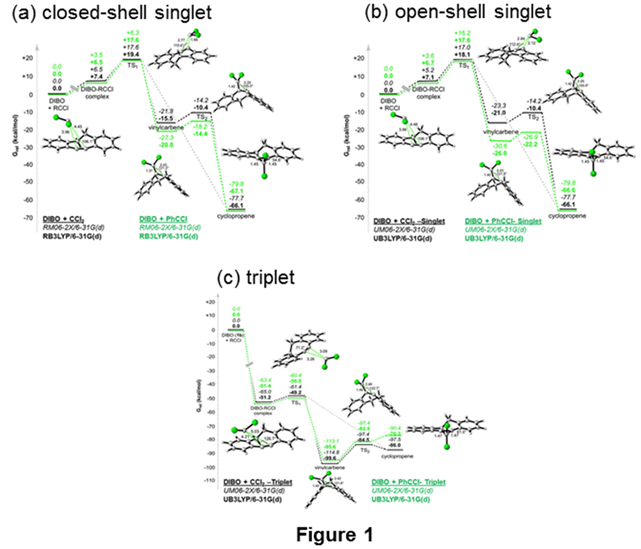
We have expanded our focus to the chlorocarbene reactivity of DIBO, 8b. We have computed the potential energy surfaces (PESs) for the additions of CCl2 and PhCCl to 8b on the closed-shell (Fig. 1a), open-shell singlet (Fig. 1b) and triplet (Fig. 1c) surfaces at B3LYP/6-31G* and M08-2X/6-31G*. ΔG‡ for RCCl + 8b is 11-12 kcal/mol, a sizeable activation barrier; very different from the barrierless addition of RCCl to 8a. Furthermore, 9b resides in a considerable energy well, making it a candidate for formation in RCCl + 8b. Evidence for this is observed in our direct dynamics trajectories.
We have calculated gas-phase direct dynamics trajectories on the closed-shell surface. The vast majority (>95%) of the trajectories lead to cyclopropene 10b-Cl2, as expected. Interestingly, we have also observed >4% of trajectories to proceed through vinylcarbene 9b-Cl2, indicating that the vinylcarbene is a viable intermediate in this addition reaction for some of the product-forming collisions.
Experimentally, we have synthesized 8b4, 5 and photolyzed it with phenylchlorodiazirine (7) at room temperature at 350 nm. We see mass spectral evidence of the addition of two equivalents of PhCCl in each of three different isomers. We are currently characterizing the structures of these isomers. We propose that the structures of these products may be butadienes 11b-PhCl which would account for three diastereomers.
This report covers the period 9/1/14-8/31/15. During this time, five Barnard College undergraduates worked on this project, three of whose summer 2015 salaries were funded by the PRF: E. Dalchand, V. Estes, A. Nadeem. One student was funded by a summer research fellowship from ConEdison (A. Urquilla). Student #5 (S. Tsuno) graduated in May 2015. E. Dalchand and S. Tsuno presented a poster on this work at the March 2015 ACS National Meeting in Denver, CO. The PI presented a poster on this work at the June 2015 Physical Organic Chemistry Gordon Research Conference in Holderness, NH.
This work resulted in a 2015 publication with 11 Barnard College undergraduates. We expect another manuscript to be submitted in the next six months on the computational results studies of RCCl + dibenzocyclooctyne.
References:
(1) Hare, S. R.; Orman, M.; Dewan, F.; Dalchand, E.; Ahmed, S.; Buzard, C.; Tolentino, J.; Sethi, U.; Terlizzi, K.; Houferak, C.; Stein, A. M.; Stedronsky, A.; Thamattoor, D. M.; Tantillo, D. J.; Merrer, D. C. J. Org. Chem. 2015, 80, 5049-5065.
(2) Tae, E. L.; Ventre, C.; Zhu, Z.; Likhotvorik, I.; Ford, F.; Tippmann, E.; Platz, M. S. J. Phys. Chem. A 2001, 105, 10146-10154.
(3) Mo, X. Y.; Bernard, S. E.; Khrapunovich, M.; Merrer, D. C. J. Org. Chem. 2008, 73, 8537-8544.
(4) Paley, D. W.; Sedbrook, D. F.; Decatur, J.; Fischer, F. R.; Steigerwald, M. L.; Nuckolls, C. Angew. Chem., Int. Ed. 2013, 52, 4591-4594.
(5) Arumugam, S.; Popik, V. V. J. Org. Chem. 2014, 79, 2702-2708.