Reports: DNI654437-DNI6: Fundamental Interactions Between Petroleum Ions and Gases
Matthew F. Bush, University of Washington
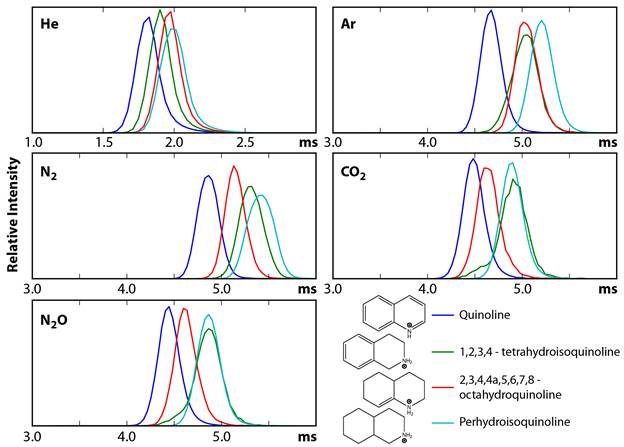
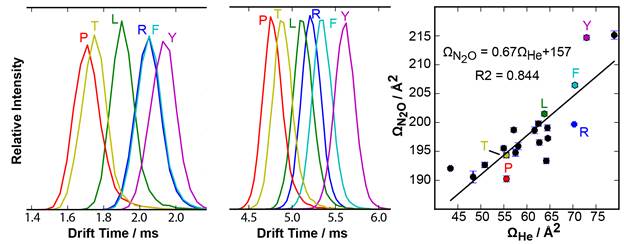
Matthew F. Bush, University of Washington


Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society