Reports: DNI753287-DNI7: Combinatorial Approaches for the Discovery of Stimulus-Responsive Supramolecular Gels
Jonathan Pokorski, PhD, Case Western Reserve University
This ACS PRF new investigator project aims to use combinatorial chemistry to identify small molecules that will undergo supramolecular polymerization in response to a given stimulus. This proposal was motivated by the many environmental catastrophes that have been at the forefront of the news recently, with the goal of sequestering environmental pollutants from large bodies of water. The main goal of this proposal was to synthesize materials that gelled in response to a given stimulus through hit-identification in a one-bead one-compound library of peptoids. In year 1, we synthesized, screened, and identified beads that were demonstrated as ‘hits’. However, peptoid sequencing was painfully slow and continues to be ongoing. In response to the difficulties in sequencing hit peptoids, we have established new polymerization catalysts that act rapidly in either water or organic solvents (i.e. phase transfer catalysts) to rapidly polymerize under biphasic conditions that would be seen in an environmental catastrophe.
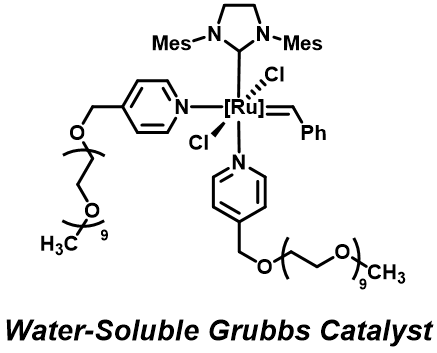
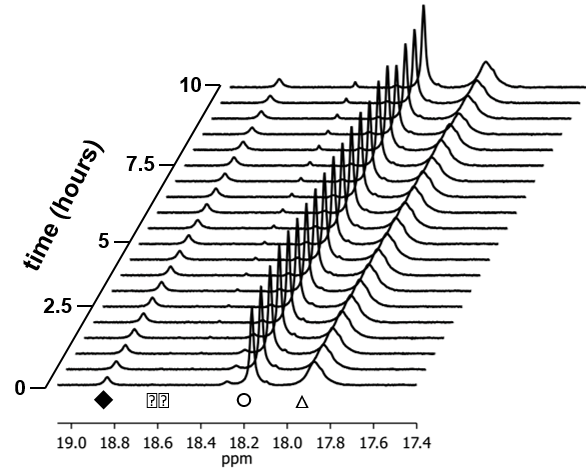
First, we sought to develop ring-opening metathesis polymerization (ROMP) catalysts that were equally stable and active under either aqueous or organic conditions. In doing so, we synthesized a Grubbs’ 3rd generation catalyst that contained short PEG groups at the pyridyl ligands (Figure 1). The water-soluble catalyst was stable under aqueous conditions for upward of 10 hours via NMR, as determined by maintenance of alkylidene signal intensity over time (Figure 2) and equally stable under organic conditions. Fortuitously, the catalyst had initiation rates between Grubbs’ 2nd and 3rd generation catalysts for ROMP using water-soluble PEGylated exo-norbornene monomers in pure D2O. This result was quite surprising, as the pyridyl ligands must dissociate from the metal center for initiation to occur. Dissociation typically requires a strongly acidic environment or competitive metal chelator (i.e. Cu). The rapid initiation is likely due to the benzyl linkage between the PEG group and the pyridine, creating a slightly less electron donating nitrogen center (compared to phenolic linkages, as previously reported by Emrick et al). By manipulating the design of the catalyst, we were able to pursue ROMP under benign conditions.
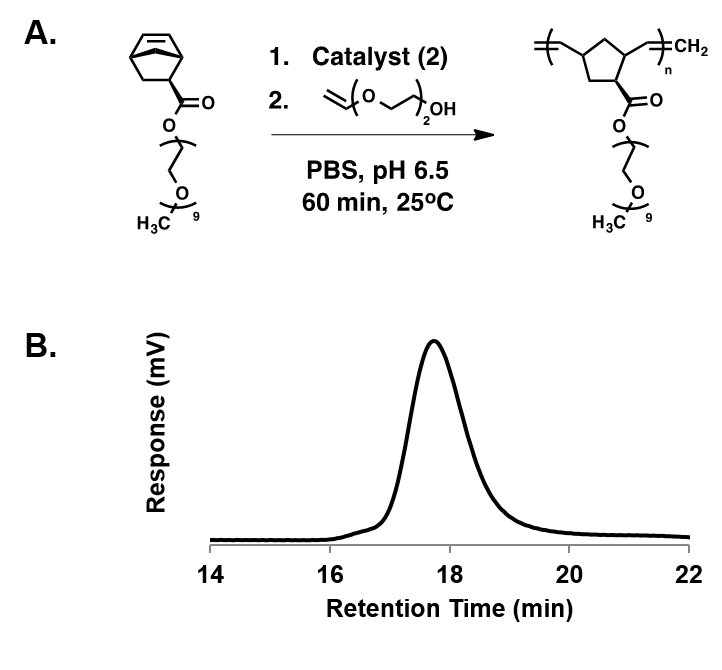
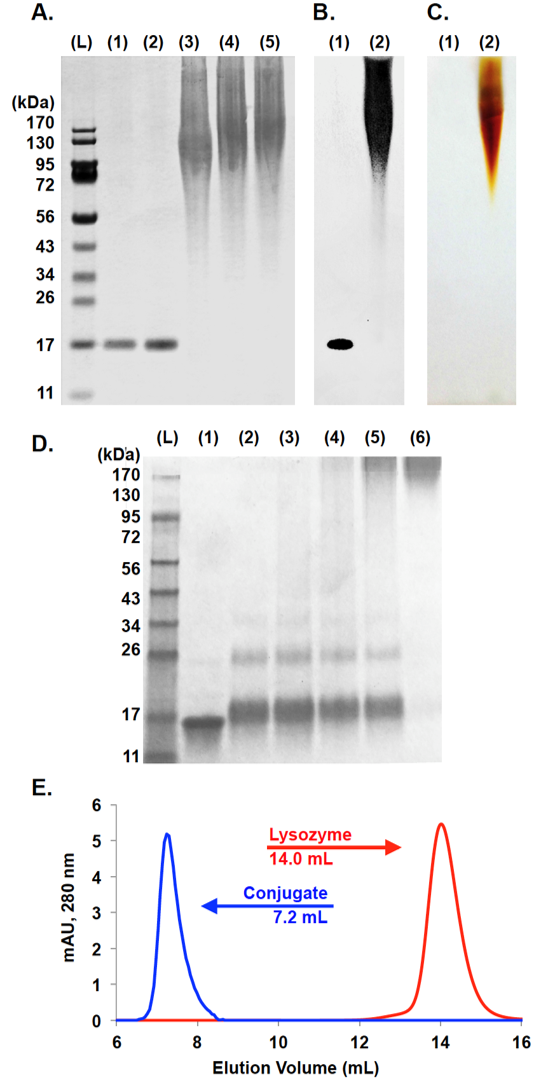
When polymerizations were performed under buffered conditions, again with PEGylated exo-norbornenes, polymerizations were complete in under an hour, thus retaining the rapid kinetics and high-fidelity of typical ROMP processes with Grubbs’ catalysts. Similarly, under organic conditions, reactions were complete in 30 minutes as confirmed by NMR. More strikingly, polymers formed during aqueous polymerization showed high molecular weight (Mn = 62.6 kDa) and very low polydispersity (PDI = 1.13) (Figure 3). The catalyst system was challenged in complex aqueous environments by docking the catalyst onto a protein through a single site insertion into a norbornyl-modified protein. From here, PEGylated norbornene was added and polymerization was completed within 30 minutes (Figure 4). The catalyst performance was outstanding in complex aqueous environments, that often times, would poison a traditional Grubbs’ catalyst. In the future we aim to polymerize multifunctional monomers at the interface between aqueous and organic to produce gels incorporating the organic pollutant. We expect that these polymerizations can be done rapidly and with high-fidelity to remove organic pollutants from aqueous sources.
Impact on Students and Career
The PRF has been a very welcome funding source in the early stages of my independent career, allowing our research group to rapidly expand and provide support for a project that was a higher risk project than others funded by start-up funds and external grants within my lab. The funding allowed me to hire a very experienced post-doc, wherein this would not have been possible off of start-up alone. By bringing Richard to the team, he has accelerated the results from my lab but more importantly he was a mentor to junior students, including undergraduates and masters students. Richard has mentored 2 undergraduates and interacted collaboratively with one master’s student on the PRF project and they have produced astounding results. Stacy Yeh received her B.S. in 2014 and made outstanding research progress over the course of this project, working closely together with her mentor. She is currently enrolled in medical school and will surely make for an excellent physician given her critical thinking skills developed within the lab (enrolled at Commonwealth Medical College). Once Stacy left, we hired Alex Prossnitz as an undergraduate researcher. In his time in the lab, he has shown an exceptional command of the research program and is rapidly helping to finish off the peptoid component of the project. Alex has been accepted to numerous outstanding graduate programs in polymer and materials science, and thus far it appears that he will be very successful in one of these top tier institutions. Lastly, Sergey Isarov joined the team to develop aqueous ROMP catalysts. He was exceptional and kick-started catalysts development in my group by undertaking extremely difficult chemistry and succeeding in a very rapid timeframe. As for my career, this has been an invaluable source of funds. By hiring Richard, this grant immediately boosted the experience level of my lab and gave me a senior peer to bounce ideas off of, provided a second source of advice for junior students, and gave a head start on research productivity.
Figure 1: Water soluble Grubbs’ 3rd generation catalyst.
Figure 2: Aqueous stability of water soluble Grubbs catalyst.
Figure 3: Polymerization under buffered condition. A) Synthetic scheme. B) GPC results, indicating high molecular weight (Mn = 62.6 kDa) and low dispersity (PDI = 1.13).
Figure 4: (A) PAGE gel of reaction mixture demonstrating kinetics; Coomassie stained. L = ladder, lane 1 = lysozyme, lane 2 = lysozyme/norbornene (1), lanes 3−5 = crude ROMP reactions terminated at 1, 10, and 60 min. (B/C) PAGE gel of purified conjugate; Coomassie stained (B)/Barium Iodine stained (C). Lane 1 = lysozyme and lane 2 = lysozyme conjugate. (D) PAGE gel of crude reaction; L = ladder, lane 1 = lysozyme/norbornene (1), lane 2 = lysozyme ROMP macroinitiator(3), lanes 3−6 = ROMP reaction with 50, 100, 200, and 400equiv of norbornene monomer (4). (E) SEC: red = lysozyme, blue =purified conjugate with 400 equiv of monomer.