Reports: UR553510-UR5: Monitoring Changes in Nanoparticle Electronics with Hammett Studies of Dendrimer Templated Supported Gold Catalysts
Bert D. Chandler, Trinity University
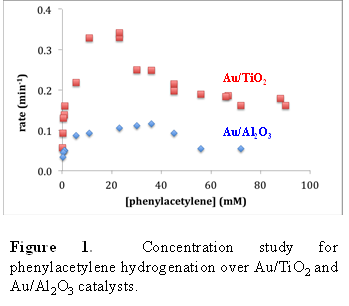
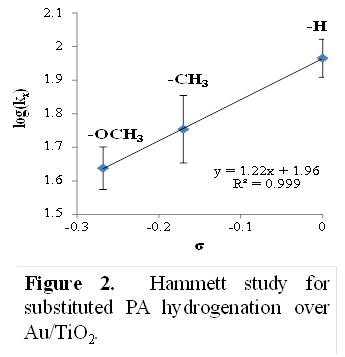
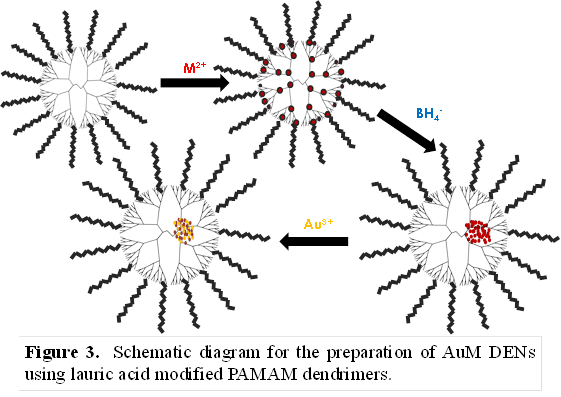
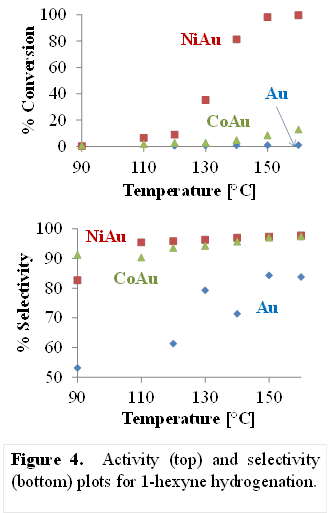
Bert D. Chandler, Trinity University




Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society