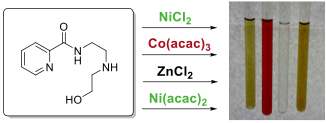Reports: UNI354581-UNI3: Novel transition metal complexes for use in asymmetric catalysis
Craig N. Streu, PhD, Albion College
Background
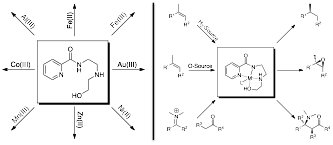
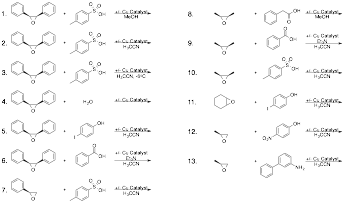
Figure 1. Proposed synthesis of chiral-at-metal coordination complexes with a tetradentate ligand (left); and examination of those chiral-at-metal complexes in catalysis (right).
Inspired by the pioneering Nobel Prize winning work of Knowles, Nayori, and Sharpless in asymmetric catalysis, and encouraged by the subsequent advances of Jacobsen and others with metallosalen catalysts, we proposed to synthesize a variety of chiral-at-metal metal complexes and test their activity as Lewis acid catalysts. The proposed synthesis is facile, and crystallography of the first of these compounds, Cu-complex 3, demonstrated an approximately square pyramidal geometry, like that of the metallosalen complexes. Given the free coordination site in this geometry, it is hypothesized that such complexes should make excellent Lewis acid catalysts. Moreover, if catalytic, these compounds would be predicted have tremendous potential for asymmetric catalysis given the ability to tune their reactivity by modifying the ligand around the central metal.
Results:
Generation of metal complexes with ligand 1-
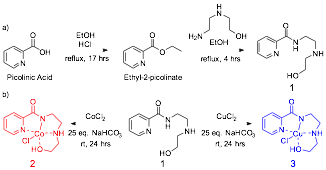
Figure 2. (a) Facile synthesis of tetradentate ligand 1; (b) Generation of highly stable cobalt (II) (2) and copper (II) (3) chloride complexes.
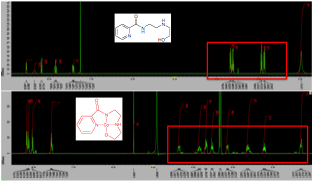
Given the success of metallosalen complexes for epoxide openings, specifically the cobalt complexes, we were especially interested in generating a chiral-at-metal cobalt complex. This red complex was produced easily using the same method that is used to produce Cu-complex 3 (Figure 2). Unlike the related copper complex, the cobalt complex 2 is diamagnetic and its formation could be monitored by the generation of diastereotopic proton signals using 1H NMR (Figure 3). Like Cu-complex 3, it is exceedingly air and water stable and was purified by flash silica liquid chromatography.
Figure 3. 1H NMR of ligand 1 (top) and Co-complex 3 (bottom) in deuterated methanol. The generation of eight diastereotopic proton signals indicates tetradentate complex formation.
A variety of metals of differing oxidation states were then used to investigate the full range of complexes that could be formed from ligand 1. Although a variety of metal complexes could be formed and isolated (Figure 4), none were as stable as Cu-complex 3 or Co-complex 2. Except for the Zn-complex, we have so far been unable to determine the three-dimensional structures of these complexes so much of our methodological work has proceeded with complexes 2 and 3.
Figure 4. Reaction of Ni(II), Co(III), and Zn(II)complexes with ligand 1 yielded markedly less stable but discrete coordination complexes that were isolated by reversed phase flash liquid chromatography.
Lewis acid behavior of cobalt complex 2 and copper complex 3-
To test the ability of these complexes to perform Lewis acid catalysis, we began with epoxide openings. This choice was made for two reasons. First, our catalysts have an open coordination site for Lewis acid coordination in much the same way that metallosalen complexes behave. Additionally, epoxide openings are preferable to Strecker reactions for work with undergraduates since they do not require the use of cyanide salts. The reactions that were performed are shown below (Figure 5). All reactions were performed with at least one aromatic reactant so that they could be easily followed by TLC. In general, reactions fell into one of two categories. In the first category the epoxides were opened in the absence of the catalyst, which was true for the aromatic epoxides with acidic nucleophiles like p-toluenesulfonic acid. In the second category, epoxides that could not be opened in the presence or absence of the catalyst, which was true for aliphatic epoxides with non-acidic nucleophiles. These results suggest that cobalt complex 2 is not sufficiently Lewis acidic to catalyze epoxide openings. Ultimately, this is may not be surprising considering the stability of these complexes to flash silica gel chromatography.
Figure 5. Reactions used to test the Lewis acid catalysis of complexes 2 and 3. Aromatic epoxides opened in the absence of the catalyst, while aliphatic epoxides were not opened by either catalyst.
Creating better Lewis acid catalysts-
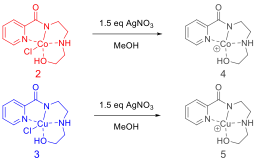
Given the inability to catalyze epoxide openings with either catalyst using the conditions above, we set out to improve the Lewis acidity of each compound. To do this, each compound was reacted with an excess of silver nitrate to remove the chloride ligand and the resulting metal complex was isolated (Figure 6). It should be noted that upon release of the chloride ligand, each complex became markedly less stable.
Figure 6. Reaction of Co-complex 2 and Cu-complex 3 with AgNO3 to remove the chloride ligand.
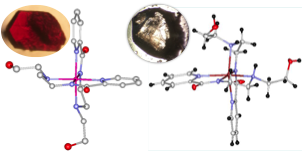
X-ray crystallography of crystals grown from the product of the cobalt complex reaction revealed the formation of an octahedral Co-complex containing two organic ligands (Figure 7). Neither of these complexes has been tested for their ability to act as Lewis acid catalysts given their octahedral geometry.
Figure 7. X-ray structures of the products formed from the reaction of Co-complex 2 with AgNO3 (left) and ZnCl2 with ligand 1 (right). Both structures reveal octahedral complexes containing two ligands.
There are essentially three major directions that we are currently pursuing to generate more Lewis acidic transition metal catalysts. First, we will examine the Lewis acidity of the less stable, but discrete metal complexes formed from Ni(II) and Co(III). Second, we are looking to generate additional metal complexes with metals of higher oxidation state. Additionally, we intend to replace the chloride ligand in both complexes with ligands like fluorine, which are likely to increase the Lewis acidity of the metal centers. Finally, we are testing the dependence of temperature on the reactivity of these complexes.