Reports: ND1052761-ND10: Mixed Transition-Metal Sulfides and Sulfo-Fluorides for Rechargeable Li-ion Batteries
Pierre F. Poudeu, PhD, University of Michigan
During the last 12 months of the project, we have completed and published the study on the "Thermal and electrochemical behavior of Cu4-xLixS2 (x = 1, 2, 3) phases"1 and have initiated a new direction focusing on the exploration of copper metal selenides as promising materials for solar energy conversion. This report briefly summarizes our progress in this new direction.
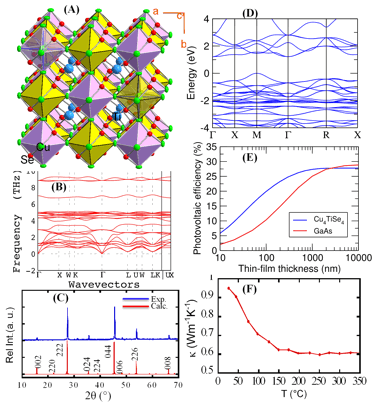
Widespread sustainable applications of photovoltaic solar energy conversion technology require the development of low-cost, high-performance materials composed of earth abundant and environmentally friendly elements. Copper-metal-chalcogenides (CMC) such as kesterite, Cu2ZnSnQ4, 2 and Cu(InGa)Se23 are among the most attractive materials for use as absorbers in thin film solar cells. However, reproducible fabrication of high performance CMC materials for various applications using current synthesis approaches has been hampered by the difficulty in controlling the density of intrinsic defects. These defects serve as dopants in the synthesized CMC materials and therefore control the electronic band bending as well as the recombination properties in solar cells. Recently, we developed an energy efficient versatile redox-triggered solid-state transformation method for the synthesis of a wide range of structurally related CMCs Cu2[M+]2[N4+]Se4 family of compounds using low cost CuSe2 as a self-sacrificial template precursor. Here we report the synthesis, structural characterization and properties of the cubic Cu4TiSe4 phase (M+ = Cu+, N4+ = Ti4+) recently discovered by our group. This new phase shows photovoltaic conversion efficiency superior to that of GaAs with excellent lattice matching to the GaAs structure. The crystal structure of Cu4TiSe4 determined using X-ray diffraction on single crystals corresponds to a new noncentrosymmetric complex cubic crystal structure (a = 11.2936 (2) , space group: F-43c, No. 229). It consists of the anionic clusters, [Cu4Se4]4- (yellow and purple octahedra on Fig. 1A) building two interpenetrating face centered cubic lattices, with the Ti4+ ions located at tetrahedral interstices. The [Cu4Se4]4- anionic cluster consists of a Cu-centered hexanuclear octahedral cluster, Cu@[Cu]6, capped by Se atoms on four of the eight triangular faces. The structure stability of Cu4TiSe4 was confirmed by calculations of elastic constants and the phonon dispersion curve (Fig. 1B). Single-phase polycrystalline powder of Cu4TiSe4 was synthesized by solid-state reactions of Cu and Ti elements with CuSe2. The single-phase nature of the synthesized material was confirmed by differential scanning calorimetry and by comparison of the measured XRD pattern (Fig. 1C) with the theoretical pattern simulated using structural data of Cu4TiSe4 obtained from X-ray single crystal structure determination. The band structure of Cu4TiSe4 was calculated with the HSE06+GW method (Fig. 1D). The material is an indirect-gap semiconductor with a gap of 1.25 eV. However, the minimum direct gap, which determines the onset of optical absorption, is only slightly larger at 1.45 eV and close to the optimal value for solar-cell materials according to the Shockley-Queisser analysis.4 What makes Cu4TiSe4 particularly promising for solar cells, however, is the large density of states (DOS) near the band edges, which gives rise to large optical absorption coefficients. The large DOS stems from the Se 4p-type and Ti 3d-type character of the valence and conduction bands respectively, which give rise to relatively flat bands (unlike the s-type conduction band of III-V semiconductors). As a result, Cu4TiSe4 is a better absorber material than GaAs for thin-film solar cells and reaches comparable efficiencies at a much lower thickness. The optical properties of Cu4TiSe4 were calculated with the BSE method and the solar-cell efficiency versus thickness was analyzed as elsewhere.5 The data shows that for a theoretical solar-cell efficiency of 25% we need a thickness of 1μm of GaAs absorber, but only 300 nm of Cu4TiSe4 (Fig. 1E), which allows the fabrication of cheaper and more flexible thin-film solar cells with Earth-abundant elements. More importantly, the lattice mismatch between GaAs (a = 5.6533 ) and a reduced cell of Cu4TiSe4 (a/2 = 5.6468 ) is only 0.1%, suggesting a possibility of epitaxial growth of Cu4TiSe4 on top of the GaAs substrate, which should facilitate the integration of this solar absorber material into current GaAs-based solar cells technology for enhanced efficiency. The compound also possesses a very low thermal conductivity of 0.6 W/m K at 350 ¼C (Fig. 1F).
References
1 Chen, E. M. & Poudeu, P. F. P. Thermal and electrochemical behavior of Cu4-xLixS2 (x=1, 2, 3) phases. J Solid State Chem 232, 8-13 (2015).
2 Jiang, C. Y., Lee, J. S. & Talapin, D. V. Soluble Precursors for CuInSe2, Culn(1-x)Ga(x)Se2, and Cu2ZnSn(S,Se)4 Based on Colloidal Nanocrystals and Molecular Metal Chalcogenide Surface Ligands. J Am Chem Soc 134, 5010-5013 (2012).
3 Todorov, T. K., Reuter, K. B. & Mitzi, D. B. High-Efficiency Solar Cell with Earth-Abundant Liquid-Processed Absorber. Adv Mater 22, 1-4 (2010).
4 Shockley, W. & Queisser, H. J. Detailed Balance Limit of Efficiency of P-N Junction Solar Cells. J Appl Phys 32, 510 (1961).
5 Shi, G. S. & Kioupakis, E. Electronic and Optical Properties of Nanoporous Silicon for Solar-Cell Applications. Acs Photonics 2, 208-215 (2015).
Figure 1: Preliminary data on Cu4TiSe4: (A) Crystal structure, (B) Phonon dispersion curves, (C) X-ray powder diffraction pattern, (D) Band structure, (E) Photovoltaic conversion efficiency of Cu4TiSe4 as a function of thickness and compared to GaAs, (F) Temperature dependent thermal conductivity.