Reports: ND1053615-ND10: Atom Probe Tomography of Kerogen and the Kerogen-Mineral Interface
Derk Joester, PhD, Northwestern University
The analysis of low-conduction materials by atom probe tomography (APT) made possible by the development of UV laser-assisted field evaporation is a very recent development. While laser-pulsing has enabled dramatic advances in our ability to visualize structure and composition of complex interfaces at the atomic scale, for example amorphous intergranular phases in nanocrystalline enamel, our understanding of the physics of field evaporation in these materials is rudimentary. We therefore conducted an in-depth study of ion-formation in fluorapatite (Fap), chloroapatite (ClAp), and hydroxylapatite (OHAp), with an emphasis on a phenomenon called multihits.
In an APT experiment, a very sharp needle-shaped specimen is brought into close proximity with a hollow cone-shaped local electrode, and a high field is applied. The tip is maintained at low temperature (typically, T<80 K). A short laser pulse is used to provide additional energy for field-evaporation, i.e. the desorption of a cation from the surface of the tip. This cation is then accelerated towards the local electrode, passes through it, and then drifts towards the detector. The mass-to-charge state-ratio (m/z) is determined from the applied field and the time-of-flight for each ion.
Unlike in metals, where most of the ions that arrive at the detector are single atoms, a substantial fraction of ions in low-conducting materials are cluster ions, i.e. ions that comprise two or more atoms. Given the relatively low mass resolving power of APT, it can be challenging to unambiguously identify these cluster ions based on their mass-to-charge state-ratio. However, many cluster ions are unstable and decay into two or more daughter ions during the acceleration period (Figure 1). Most daughter ions differ in their m/z, and their m/z is also different from the parent ion. Consequently, they are accelerated differently, arrive at the detector a slightly different times, and are identified as ‘multihit’ events. Multihits can account for a significant fraction of overall hits in an insulating material, for instance ~19% in apatites. Given that multihits are typically cluster ions, the fraction of all ions that are detected in multihits is at least double that, and often considerably larger. It is therefore very important to improve our understanding of multihit statistics, cluster ion stability, and decay pathways. While our current approach is largely descriptive, we are hopeful that this data will provide a basis, and ultimately a benchmark, for simulations of the field evaporation process in low-conducting materials.
Figure 1. Schematic representation of a dissociation event during the acceleration phase of an APT experiment.
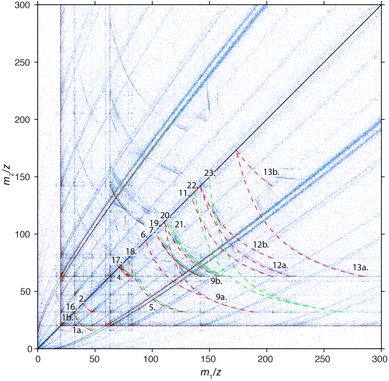
One way to analyze multihit events is by constructing a correlation histogram. Correlation histograms report the frequency at which combinations of daughter ions (binned by their m/z) occur in an APT data set. We constructed correlation histograms for multihits of multiplicity 2, i.e. for the case that a decay results in exactly 2 daughter ions, from APT data sets generated from synthetic single crystals of FAp (Figure 2), ClAp, and OHAp.
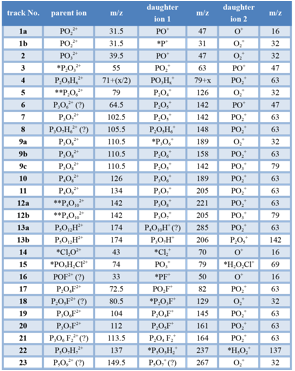
Figure 2. Correlation histogram of fluorapatite (FAp). Simulated dissociation tracks unique to FAp are indicated in green, tracks observed in all endmember apatites are indicated in red. Specific dissociation reactions are listed in Table 1.
Dissociating
cluster ions were then identified by a feature in the correlation histogram
called dissociation track. A simple physical model relates the apparent
(observed) mass-to-charge state-ratios of the daughter ion (m1'![]() ) to the actual
mass-to-charge state-ratios of parent (mp) and daughter ions (m1,m2)
) to the actual
mass-to-charge state-ratios of parent (mp) and daughter ions (m1,m2)
, using the fractional potential drop Vd/V0 as a parameter. The fractional potential drop is zero when the cluster ion dissociates at the surface of the tip (corresponding to the right ‘end’ of the individual tracks in Fig. 2), and 1 if it dissociates at the very end of the acceleration path (corresponding to the left end of the track). Careful analysis of the correlation histograms of apatite endmembers allowed us to identify a number of cluster ions and their decay products (Table 1). Assignment of the cluster identities and the decay routes is a non-trivial task because experimental dissociation tracks are noisy and incomplete. While there are nearly always several possible assignments, we developed a range of criteria to select the most probable candidates.
Table 1. Dissociation reactions observed in multihits with multiplicity 2 in APT data sets of FAp, ClAp, and OHAp. Annotation: * not seen in single hit spectra; ** ambiguous identification.
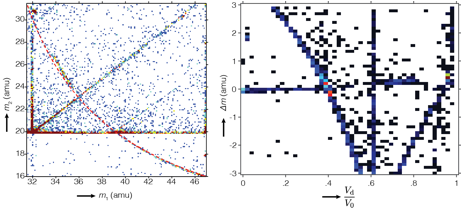
A detailed analysis of individual dissociation tracks further revealed that their structure, i.e. the number of dissociation events as a function of the fractional potential drop, which is a measure of distance from the tip or time after evaporation at which the dissociation occurs, defied simple models such as a simple exponential decay. Furthermore, there appear to be systematic deviations of the experimental dissociation tracks from ones predicted by the simple model (Eq 1). To improve visualization and allow for a quantification of deviations from the theoretical model, we developed a routine to straighten dissociation tracks (Figure 3).
Figure 3. The dissociation track for one of the dissociation reactions of PO22+ is shown here as it appears in a regular correlation histogram (A), and after straightening by a coordinate transformation (B). Note that there is a narrow window during which a large number of dissociation events seem to occur (arrow).
We are currently in the process of converting the fractional potential drop into a more intuitive scale, such as distance from the tip, or time after evaporation. This requires a number of relatively straightforward finite element calculations. However, my group has practically no prior experience with finite element modeling. As a consequence, this step has taken a little longer than anticipated. Nevertheless, a manuscript describing our findings is in an intermediate draft stage, and I expect we will be able to submit by mid 2016.