Reports: UR454244-UR4: Determining the Significance of Thermally-Activated Heavy-Atom Tunneling in Diradical-Forming Concerted Reactions (Cycloaromatizations) of Pertinence to Organic and Physical Chemistry
Edyta Greer, PhD, Baruch College
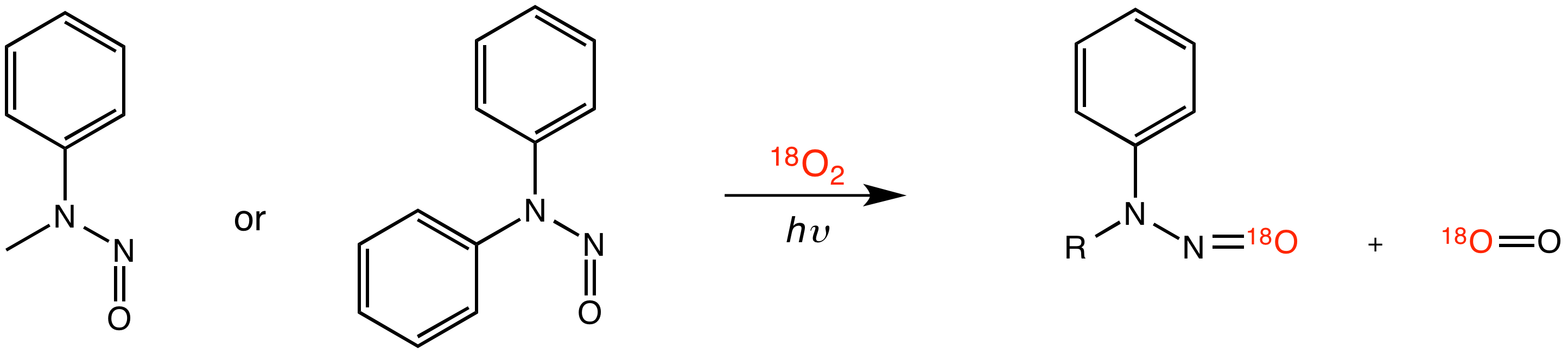
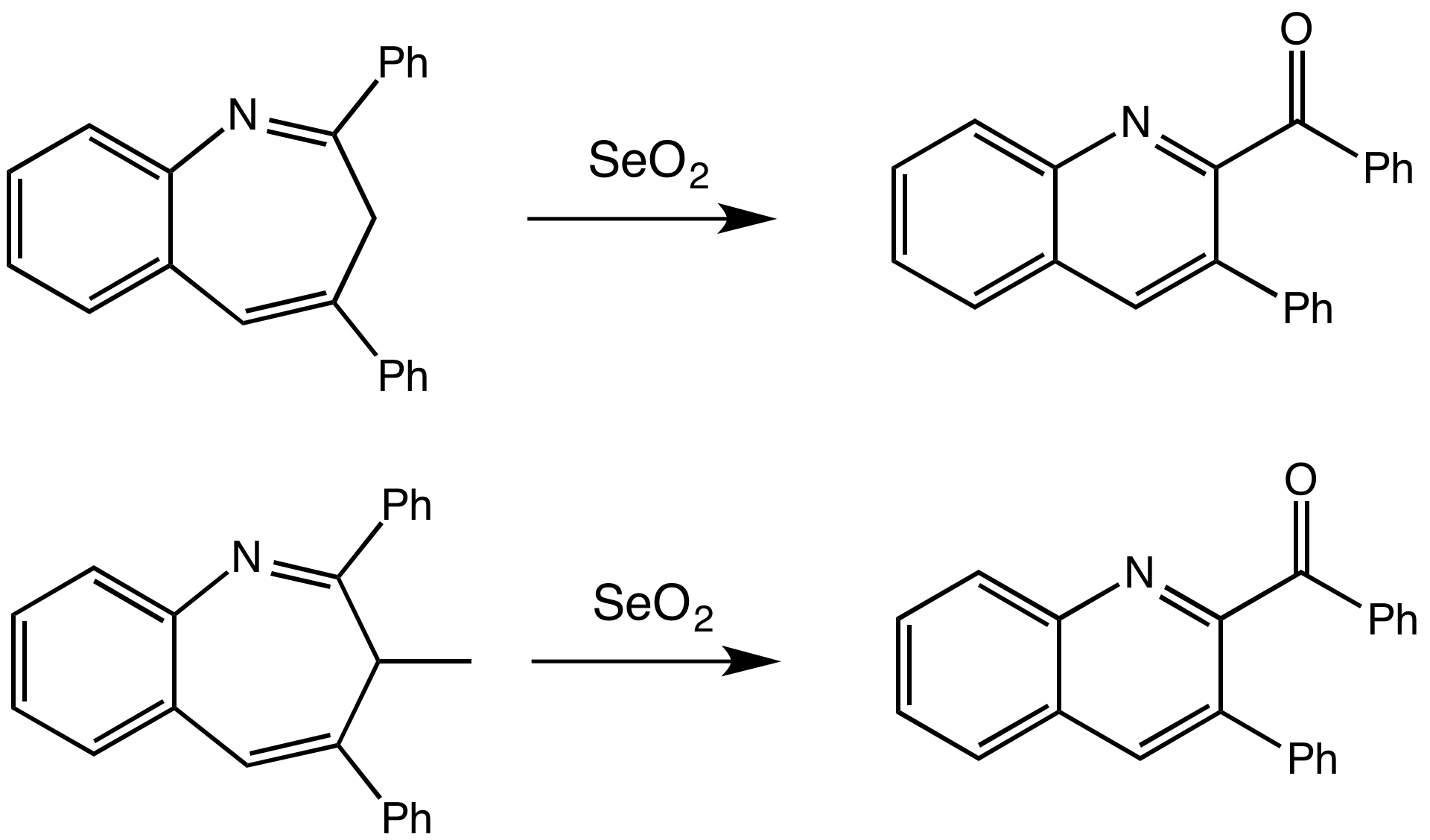
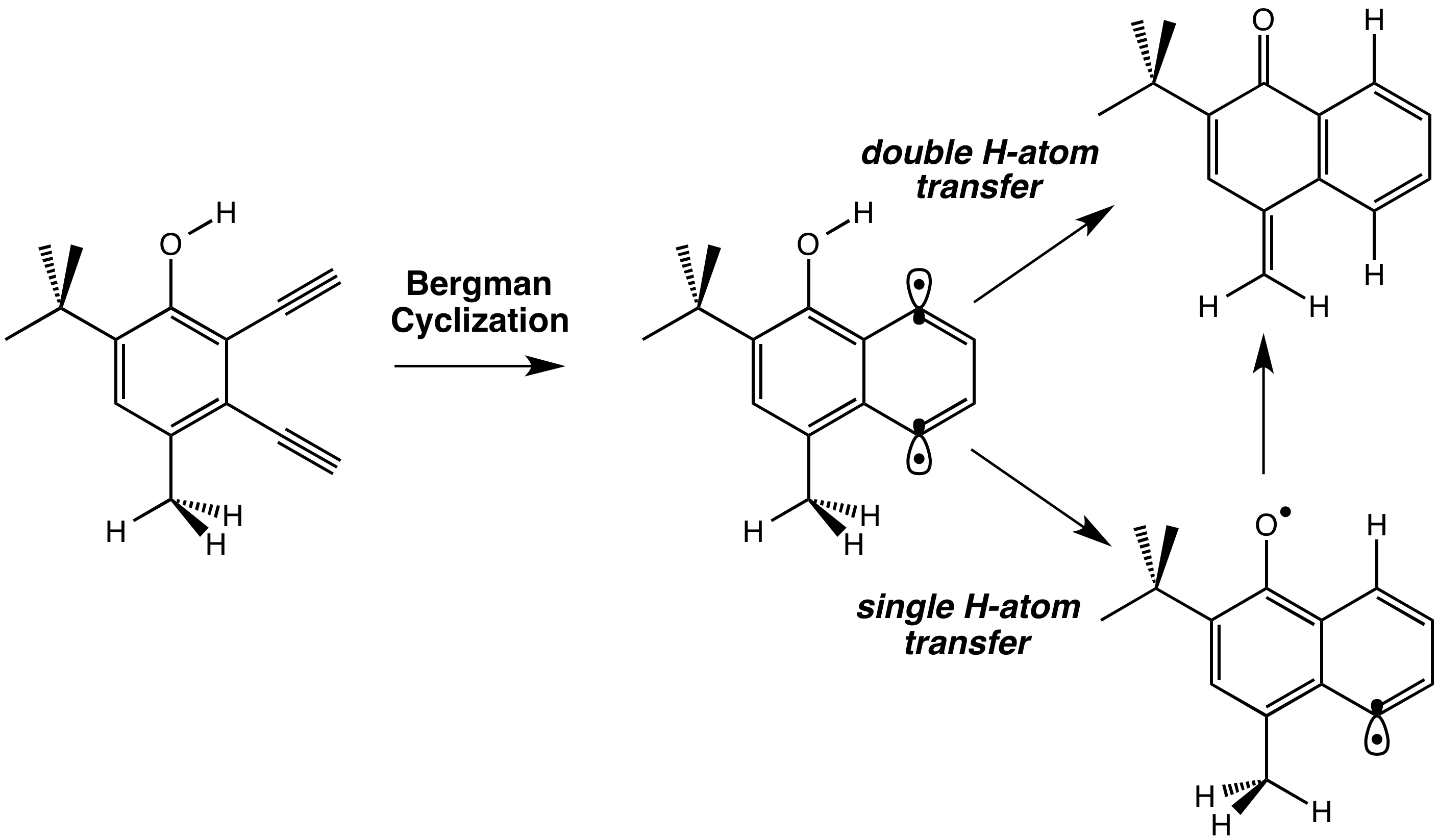
Edyta Greer, PhD, Baruch College
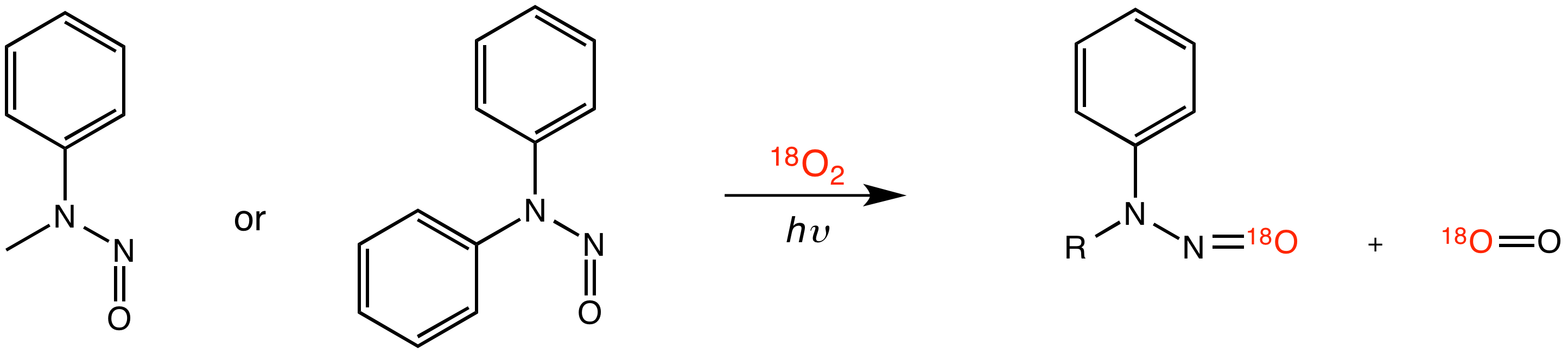


Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society