Reports: UR754033-UR7: Development of Supramolecular Polymers Based on Novel Methylene-Bridge-Linked Multi-Calixarenes
Jordan L. Fantini, PhD, Denison University
Figure 1. Figure 3. Figure 4. Figure 5.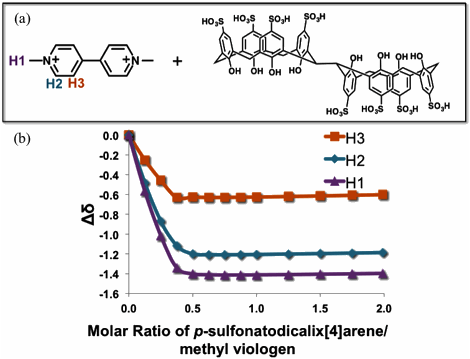
Figure6.
Jordan L. Fantini, PhD, Denison University
Figure 1. Figure 3. Figure 4. Figure 5.
Figure6.
Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society