Reports: UNI153730-UNI1: Synthesis of Highly Substituted Piperidines from Petrochemical Compounds
Jenny M. Baxter Vu, PhD, Valdosta State University
Introduction
The construction of complex, versatile molecules from unsaturated hydrocarbons, abundant in petrochemical feedstock, is highly desirable. While cycloaddition reactions of 1,3-dipolarophiles with dienes has also been reported in the literature, the full potential of this transformation has not been exploited. This work reported herein focuses on the [3+2] cycloaddition reaction between aryl nitrile oxides and isoprene. Various conditions for the generation of nitrile oxide from either the oxime or the chloroxime were investigated to determine the effect of reaction conditions on regioselectivity of the products. The effects of electronic turning of the aryl oxime on the selectivity of the [3+2] cycloaddtion reactions are also reported herein. Although the current work only discusses the results of the cycloaddition reaction itself, efforts toward converting the products of these reactions into biologically active molecules will be the focus of future investigations in our research lab.
I. Electronic modification of aryl nitrile oxides in [2+3] cycloaddition reactions with isoprene
The first goal of this project is to explore the selectivity of the [2+3] cycloaddtion reaction of an aryl nitrile oxide with an unsymmetrically substituted diene. Therefore, we began by investigating the effect of reaction conditions used to generate phenyl nitrile oxide in situ and electronic tuning of the aryl nitrile oxide on the selectivity and yield of the product.
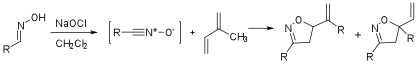
We explored two ways of generating the phenyl nitrile oxide in situ: oxidation of stable aryl oximes and the treatment of chloroximes with a base. The oxidation of phenyl oxime (1a) was accomplished either by treatment of a 3% aqueous solution of NaOCl or via the addition of PhI(OAc)2 to a solution of diene in methylene chloride. The biphasic reaction of aqueous NaOCl with isoprene at 0°C resulted in moderate yields (53-75%) with ~3:1 selectivity while the use of PhI(OAc)2 at 0°C as an oxidant provided products with less selecitivity (1.7:1, 95% conversion at 16 hours, yield TBD). Although the reaction with PhI(OAc)2 was significantly slower at -20°C, the selectivity was further attenuated (~1:1, 20% conversion at 26 hours). These results are summarized in Table 1.
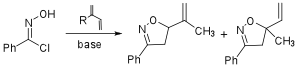
Although the treatment of an oxime with NaOCl to form a nitile oxide most likely involves a chloroxime intermediate, we also explored the direct formation of phenyl nitrile oxide from the reaction of the corresponding chloroxime and a variety of bases in the cycloaddtion reaction with isoprene. These base screening results are shown in Table 2 and included amine bases (entries 1, 4, and 6), hydroxide and alkoxide bases (entries 2-3) and iPrMgCl (entry 5). The selectivities ranged from 2.4:1 (Hunigs base, entry 1) to 4.1:1 (Et3N, entry 6) and were determined by either HPLC assay or 1H-NMR.
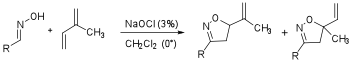
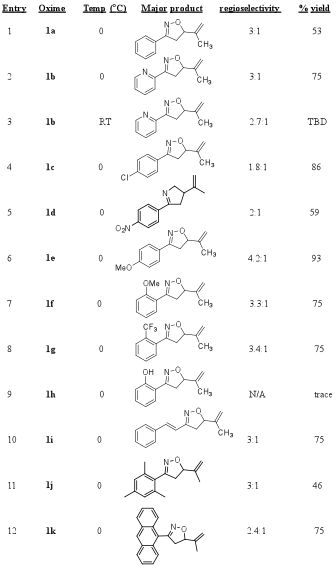
We hypothesized that the stability of the nitrile oxide may be an important factor in the observed regioselectivity in the products from the [2+3] cycloaddtion reaction of an aryl nitrile oxide and isoprene. A less stable nitrile oxide would perhaps react faster with less selectivity while a stabilized nitrile oxide would prefer the less hindered alkene moiety and provide higher selectivity in the products. To test this hypothesis, we performed the [2+3] cycloaddtion reaction of various aryl oximes, isoprene, and aqueous NaOCl at 0oC to provide the corresponding vinyl-substituted isooxazoline products (5 and 6). These results are shown in Table 3. The aryl (Ar) oxime substrates in the reaction included both electron withdrawing and electron donating groups relative to the standard phenyl oxime (Ar = Ph), which resulted in a 3:1 selectivity. Some noteworthy results from this screening are included in the following discussion: Entry 2 (Ar = 2-pyridine) shows that this minor change provides the same selectivity (3:1) but entries 4 and 5, Ar=p-Cl and p-N2O respectively, each result in a lower selectivity of 1.8:1 and 2:1. Consistent with our hypothesis the electron-donating groups (p-OMe, entry 6 and o-OMe, entry 7) show improved, albeit only slight, selectivities (4.2:1 and 3.3:1). Entries 7 (o-OMe) and entry 8 (o-CF3) suggest that groups in the ortho- position have little to no effect on the selectivity of the reaction. This conclusion is further supported by the observed selectivity (3:1) in entry 11 (Ar = mesitylene). While we have not yet obtained the desired regioselectivity (>10:1), the regioselectivites in these reactions lend support for our hypothesis and suggest that stability of the nitrile oxide is an influential factor in the selectivity of products from the [2+3] cycloadditon reaction.
II. Steric modifications to dienes for [2+3] cycloaddition reactions with phenyl nitrile oxide
Our investigations on developing methodology for the highly selective synthesis of vinyl-substituted isooxazolines via a [2+3] cycloaddition reaction between nitrile oxide and diene suggest that only moderate fluctuations in product selectivities can be obtained via the reaction conditions or electronic tuning of the nitrile oxide. Therefore, we will turn our attention to the steric and electronic tuning of the diene (see Figure 1). Our future efforts will focus on the synthesis of a variety of non-symmetrically substituted dienes to use as substrates in the cycloaddition reaction.
Figure 1. Electronic tuning hypothesis for diene
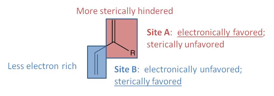 Table 1. Cycloaddition reactions with phenyl oxime and isoprene
Table 1. Cycloaddition reactions with phenyl oxime and isoprene
1a 4 5 6
|
Entry |
Temp. (°C) |
Time |
Ratio of 5:6 |
% Conversion |
|
1 |
0° |
16 hrs |
1.7:1 |
>95% |
|
2 |
-20° |
4.5 hrs |
- |
trace |
|
3 |
-20° |
26 hrs |
1:1 |
̴20% |
Table 2. Cycloaddition reactions of chloroxime 2 and isoprene with various bases
5 6
|
Entry |
Base |
Temp. (°C) |
RSa |
% yielda |
|
1 |
N(i-Pr)2Et |
RT |
2.4:1 |
75 |
|
2 |
KOtBu |
RT |
2.7:1 |
20 |
|
3 |
NaOH |
RT |
2.3:1 |
TBD |
|
4 |
DMAP |
RT |
3.1:1 |
TBD |
|
5 |
iPrMgCl |
0 |
2.6:1b |
TBD |
|
6 |
Et3N |
RT |
4.1:1b |
TBD |
Table 3. Cycloaddition reactions of various oximes with isoprene
1a-k 4 5a-k 6a-k