Reports: ND954593-ND9: Confinement Effect on Solubility of Oilfield Fluids in Sub-Micron Pores
Keith E. Gubbins, PhD, North Carolina State University
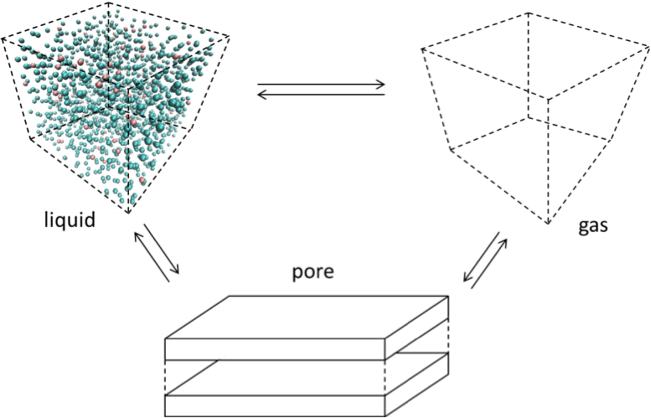
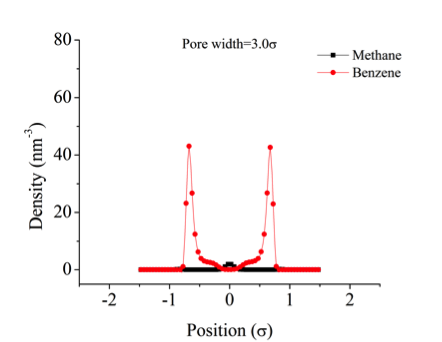
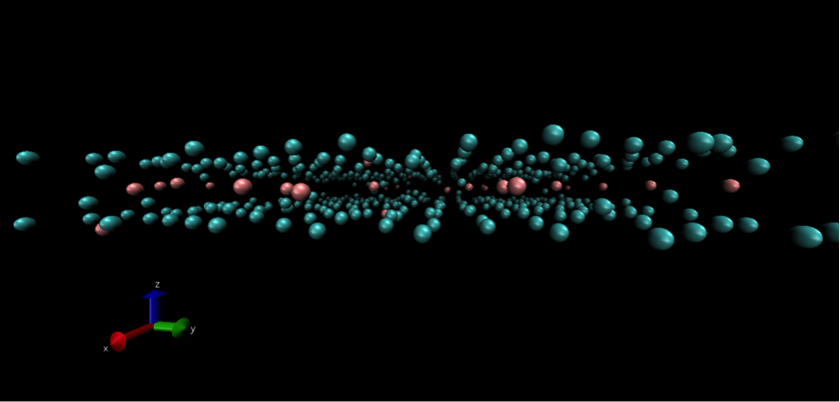
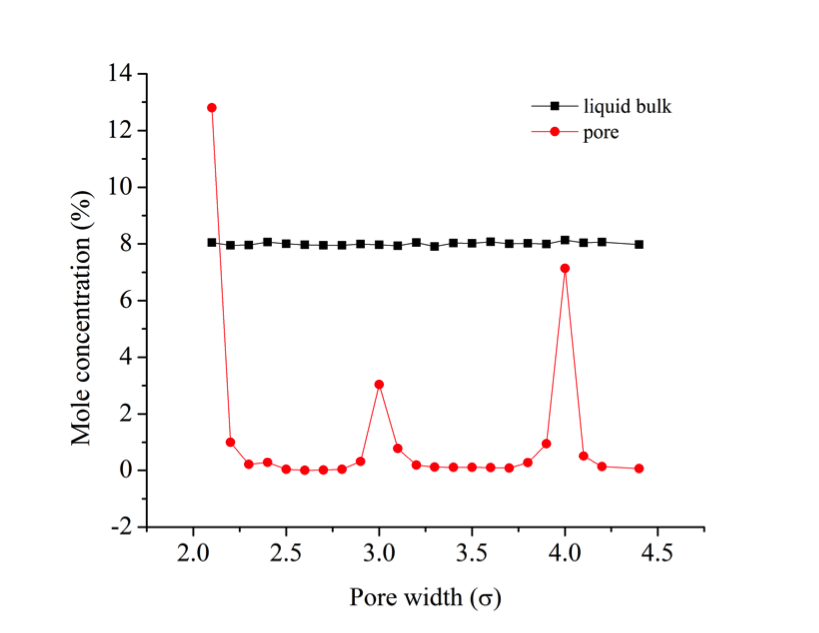
![]()
![]()
Keith E. Gubbins, PhD, North Carolina State University




![]()
![]()
Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society