Reports: UR152993-UR1: Aromatic Donor-Acceptor Organocatalysis: Noncovalent Activation of Aryl Halides in Green Palladium Cross-Coupling Reactions
Joseph J. Reczek, PhD, Denison University
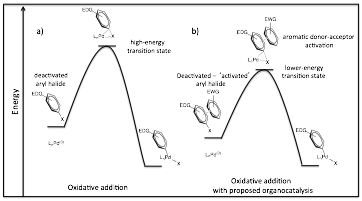
In the past year we have continued our work towards developing a general approach to the aromatic donor-acceptor organocatalysis of electron-rich (deactivate) aryl chloride coupling reactions. The overall plan is to lower the activation energy of the rate limiting oxidative addition step in these reactions by adding an electron-poor aromatic co-catalyst. In aqueous solvents, the complementary electrostatics of the aromatics will lead to face-centered stacking (solvophobic effect), possibly decreasing the C-Cl bond strength of the aryl chloride and driving the reaction (Figure 1).
Figure 1. Illustration of the first oxidative addition step in aryl halide cross-coupling reactions, highlighting the transition state energy for: a) a standard aryl halide palladium coupling, b) the proposed ADA-activated aryl halide coupling.
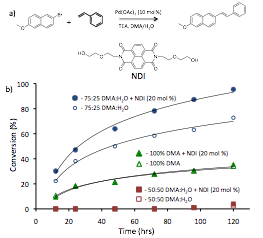
In initial studies, we observed a modest rate enhancement of a Heck coupling reaction in the presence of an electron-poor naphthalene diimide (NDI) co-catalyst (Figure 2). While promising, reaction times in this system were not conducive to timely study of conditions. Additionally, the poor water miscibility of the reagents (no reaction observed at 50% water) limited the solvophobic driving force for potential ADA catalysis that could be leveraged for this system.
Figure 2. a) Scheme for Heck reaction with NDI co-catalyst. b) Graph of GC-MS data following % conversion of six Heck reactions with and without the co-catalyst.
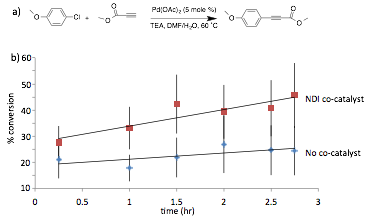
We next moved to exploring ADA catalysis in Sonogashira coupling reactions, which generally have significantly shorter reaction times compared to the similar-type Heck reactions. In the presence of the same NDI co-catalyst, we found conditions in which a rate enhancement trend was observed for the Sonogashira coupling in a reasonable time frame (Figure 3). However, solubility is still limited in this system, and the % conversion measured showed a high degree of variability, leading us to believe that we were not collecting homogeneous reaction samples.
Figure 3. a) Sonogashira coupling reaction initially explored. b) Percent conversation with and without NDI co-catalyst. The data sets shown are the average of 5 independent reactions under the same conditions. While promising, error bars indicate the high level of variation in our system, and limit the statistical significance of the observed rate enhancement.
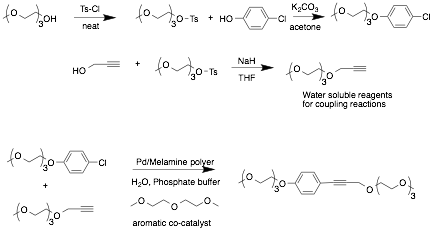
In the past year we have focused on the synthesis of model reagents with high solubility in water in order to maximize solvophobic interactions and increase the consistency of our reactions set up and analysis (Scheme 1a). We have been able to synthesize gram quantities of the PEG-appended aryl chloride and alkyne shown below, and have begun studies on the ADA assisted coupling of these reagents in 100% water as solvent (Scheme 1b). While these reagents were soluble in water, we unfortunately found that the Pd(OAc)2 catalyst was not compatible with these reaction conditions, giving highly inconsistent results in both control and co-catalyzed reactions.
We then turned to trying a variety of Pd catalysts, some of which we synthesized in house. At the end of this reporting period in July were successful in finding a catalyst system that worked consistently for our reagents in 100% water. The catalyst is made by first generating a poly-melamine scaffold to which Pd is complexed. The result is a polymer-supported catalyst that is completely water soluble and able to consistently give high yields in our Sonogashira couplings. We are now investigating a variety of electron-poor aromatic co-catalysts to enhance the rate of these reactions.
Scheme 1.
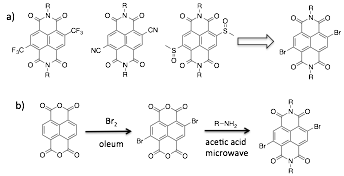
As we continue to develop model reaction set-ups to illustrate general ADA organocatalysis, we have also been working towards the syntheses of electron-poor aromatic catalysts other than NDI. This includes core-substituted NDI (cNDI) derivatives with greater electro-positive surfaces (Figure 4a). These cNDI derivatives are all derived from a common intermediate, the corresponding dibromo-NDI. Surprisingly, when we began working on the target cNDI ADA catalysts, we found that there was no good synthetic procedure available for the key dibromo-NDI intermediate, with literature preps giving actual yields from 12-15%. This led us to develop an optimized synthetic procedure for the dibromo-NDI (Figure 4b). By monitoring the brominating with 1H NMR, and using concentrated microwave heating for the imidization, we were able to more than double the efficiency of isolating pure dibromo-NDI. This work was published this year in Synthetic Communications.
Figure 4. a) Target core-substituted NDI derivatives to serve as ADA catalysts. All derived from the dibromo-NDI intermediate. b) Scheme for the optimized synthesis of dibromo-NDI.
Impact:
In addition to the progress discussed above, this PRF funding has had a significant impact on Denison's department of Chemistry and Biochemistry. This grant has funded a student, Michelle Hill, in full-time undergraduate research for the summer of 2015. Michelle, now a junior at Denison, is an African American woman who has decided to pursue a graduate career in chemistry. She will be continuing her work on this research in 2015-2016, including a second summer of full-time research. Michelle will also be presenting her work at the 2015 ABRCMS meeting. This funding has also contributed to chemicals and supplies for Michelle and two other undergraduate students participating in semester-research for the past year. Importantly, this grant has made it possible for me to recruit Michelle as a sophomore to do research, and I intend to recruit two more sophomores in the coming year to engage on this project. This significantly enhances the research culture of the department at Denison, raises the visibility of the chemical sciences among students, and enhances the likelihood of students continuing in the sciences. Finally, this funding has helped provide the resources for the recently published publication mentioned above. On behalf of my students, and myself, I am thankful to PRF for the invaluable support of undergraduate research.