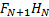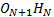Reports: ND753980-ND7: The Modular Synthesis and Fundamental Properties of Hyperbranched Polymers Based on bis-MPA Monomers: Comparison with Dendrimers and Linear Analogs
Scott M. Grayson, Tulane University
I. Project Goal: The overarching aim of this proposal is to determine how branching effects the physical properties of hyperbranched polymers. Although hyperbranched polymers have been studied in tremendous detail, they have never been compared side-by side with exact linear and dendrimer analogs. The bis-MPA monomer is one of the most important for commercial applications of hyperbranched polymers, and therefore we are setting out to develop the synthesis of exact dendrimers, linear polymers, and pseudodendrimers.
II. Synthetic Research Progress:
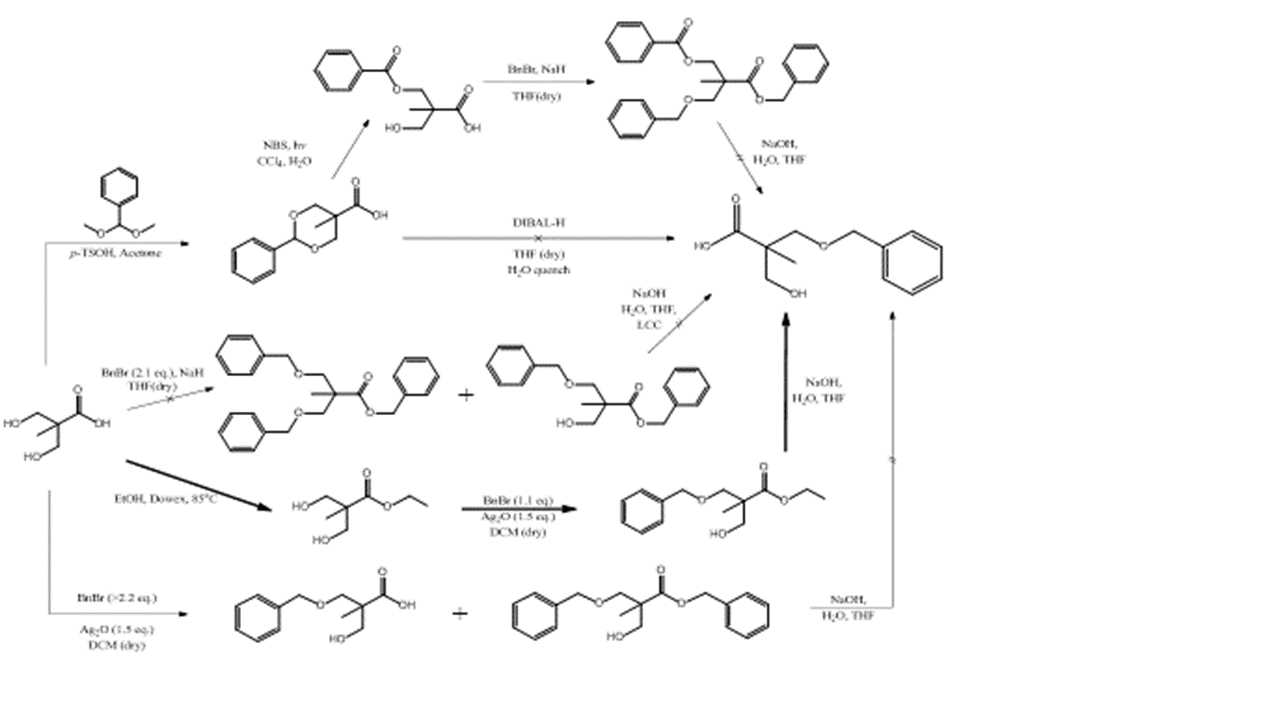
a) Linear Analogs: Although the hyperbranched and dendrimer analogs of bis-MPA have been reported, the linear analogs have not. The most apparent route for making linear bis-MPA polymers is condensation of a mono-protected monomer. A number of routes have been attempted:
Scheme 2.1: Multiple synthetic routes attempted to yield linear bis-MPA monomer
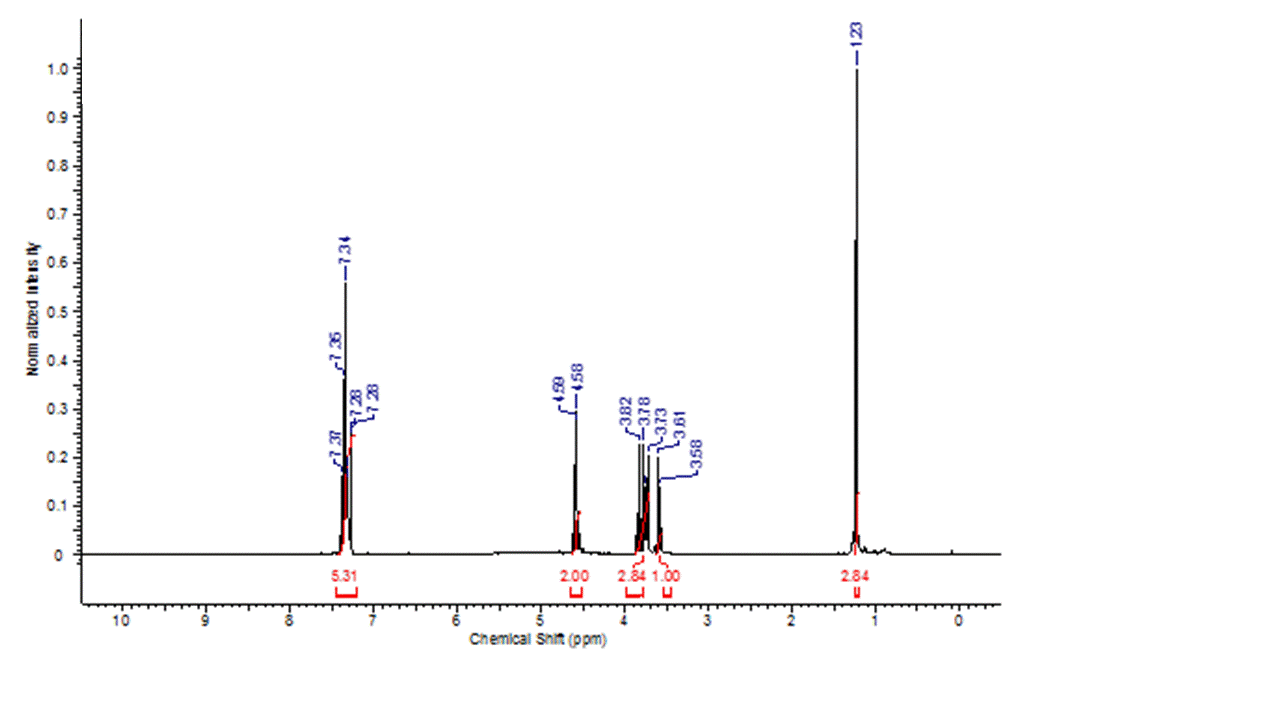
Although multiple routes proved effect to produce the desired linear monomer, the lower route that utilized silver (I) oxide as a catalysts for the selective monobenzylation. This route proved to be highly efficient, and generated the desired product with exceptional purity. The 1H NMR of this compound is show in figure 2.1.
Figure 2.1: 1H NMR of the monobenzylated bis-MPA monomer confirms the exceptional purity of the compound isolated from this route.
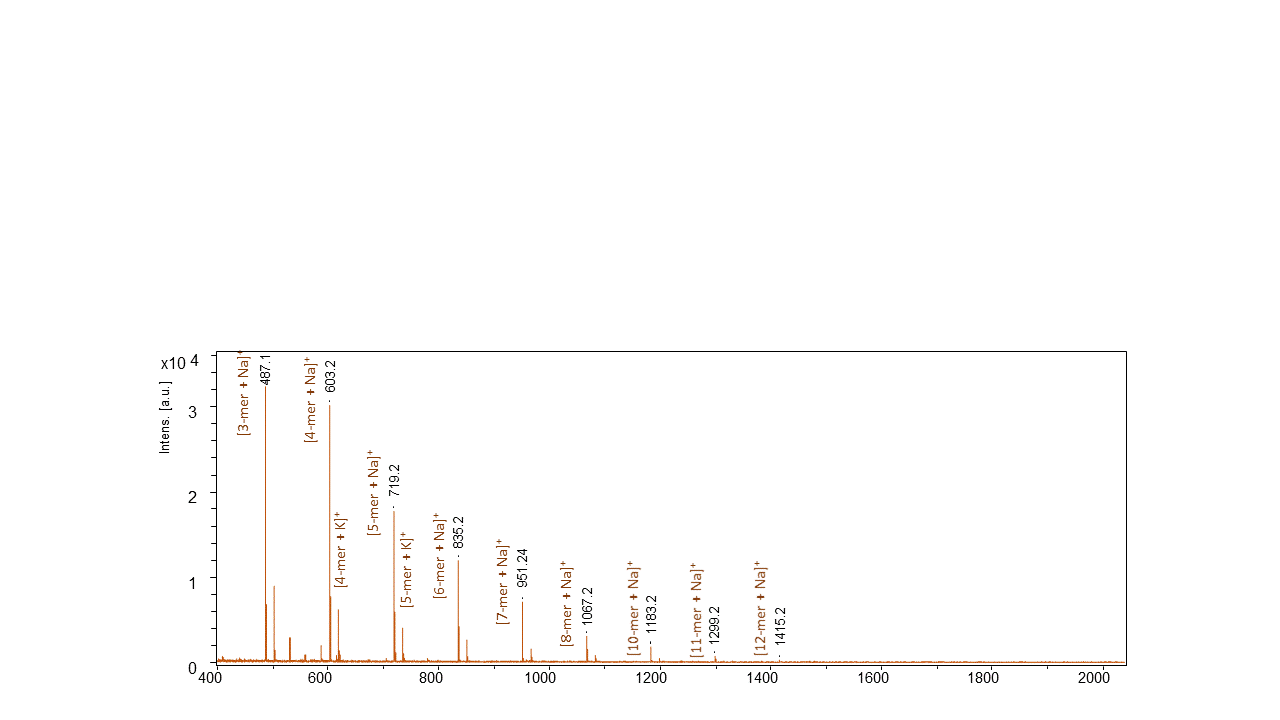
2b) Hyperbranched Analogs: While the synthesis of hyperbranched bis-MPA polymers has been well-established, the proposed kinetic studies require that the hyperbranched polymers be grown from specific cores. Our initial target has been to make heptanol initiated hyperbranched polymers. Protocols for preparing have been optimized, and a number of low molecular weight batches of hyperbranched bis-MPA have been prepared, including an examples if figure 2.2.
Figure 2.2: Hyperbranched bis-MPA generated by initiation off of heptanol. Note the major distribution is the sodium adduct, and the secondary distribution the potassium adduct.
2c) Pseudodendrimer Analogs: Pseudodendrimers of bis-MPA are being prepared via reaction of hyperbranched polymers with a benzylidene-protected bis-MPA anhydride. Initial results confirm these reaction can undergo reaction, but that they take some time to react in a quantitative fashion. Optimization of this reaction is ongoing.
2d) Dendrimer Analogs: The bis-MPA dendrimers have already been reported by others, and there synthesis has been scaled up, so as to provide material for physical studies.
III. Characterization Research Progress:
While bis-MPA based dendritic polyesters have rapidly become commercially important materials, many of the critical factors that define the relationship between their structure and bulk physical properties have yet to be fully understood. The bulk properties of bis-MPA dendritic polymers are largely governed by the ability of these molecules to form hydrogen bonds (H-bonds), both intra- and intermolecularly, primarily via association of the hydroxyl groups with each other and/or with carbonyl, ester, and ether oxygen atoms. The main objective of the past year study was to explore experimentally and computationally, via molecular dynamics (MD) simulations, the H-bond organization and interrelated structural order formation in these polymers.
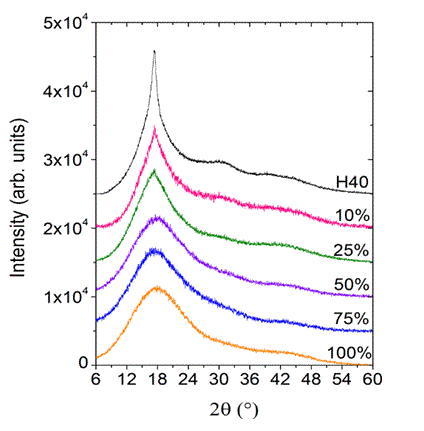
Fig. 3.1 WAXS patterns of Boltorn® H40, and 10, 25, 50, 75 and 100% esterified H40.
3a) WAX characterization: An addition to the amorphous halo peak at 2θ ≈ 17°, which is typically observed for hydrocarbon compounds, the WAXS intensity patterns of bis-MPA based hydroxylated dendrimers and hyperbranched polymers (shown in Fig. 3.1) exhibited a minor peak at 2θ ≈ 30°. The peak at 2θ ≈ 30° attracted our attention, in regards to its structural origin, because it was reminiscent of the WAXS intensity maximum observed in liquid water. It is therefore hypothesized that this corresponds to extended networks of hydrogen bonding in the hyperbranched polymers. Ongoing studies with a range of different dendrimers and hyperbranched polymers are designed to elucidate this relationship.
3b) Computational Modelling: The simulated structures revealed H-bonded ON+1HN ‘chain-like’ clusters of different lengths, which ranged from single H-bond associations to clusters containing eight oxygen atoms. Approximately 50% of all oxygens involved in H-bonding were part of the longer clusters. The generation number did not affect the degree of cluster formation considerably. The irregularly branched HBP, H20, however, displayed a higher propensity to form single H-bonds as compared to the dendrimers. The predominant type of H-bond association in the simulated systems was between hydroxyl groups (OH∙∙∙OH) while the OH∙∙∙O=C type association was the second most important. Morphologically, the H-bonded ‘chain-like’ clusters discovered in hydroxylated dendritic polymers resembled the H-bonded FN+1HN ‘chain-like’ clusters previously observed in liquid hydrogen fluoride.
The MD simulations also enabled us to expose the globular shape of the dendritic structures in the bulk and to investigate to what extent the core can influence the dendrimer shape. Our preliminary computational studies suggest that shape is extremely important in hydrogen bond interactions, at least at lower generations.
IV. Remaining Challenges and Future Research Directions:
Synthesis of Additional bis-MPA Analogs: With the synthesis of selectively protected monomers established, the remaining synthetic goal is to synthesize and characterize a large library of polyesters, including, linear, hyperbranched, pseudodendrimers and dendrimers. These will be used to inform the role of branching and structure in defining the physical properties of polyesters based on architecture. In addition to characterization by WAXS and additional computational modeling, remaining research efforts will also focus on one-pot kinetic studies. By using mixtures of the above materials the relative rates of coupling, the relative “architecture-dependent” rates of coupling can be confirmed.
V. Research Impact: While it is well known that architecture affects physical properties and rates of reactivity, there have been no systematic studies of multiple architectures based on the same monomer to systematically elucidate these architectural effects. In addition to providing a critical systematic study, this study aims to establish new techniques for probing polymer functionalization kinetics, based predominantly on MALDI-ToF mass spectrometry.
VI. Impact on Researchers and Collaboration: The generous funding by the ACS-PRF through the “type ND program” has been a vital part of establishing a new collaboration between the two co-PIs with complementary research capabilities. It has also enabled the research support of multiple graduate students to enable them to focus more aggressively on their research portfolios and expand their interdisciplinary training.