Reports: UNI153245-UNI1: The Use of Terminally-Functionalized, Atactic-Polypropylene Oligomers as Supports for Homogeneous Catalysis
Christopher E. Hobbs, PhD, Texas A&M University-Kingsville
As the title of the grant suggests, the goal of the Hobbs research laboratory has been the utilization of soluble polypropylene (PP) as a new support for synthesis and catalysis. This chemistry was extended to the preparation of isotactic-poly(propylene-co-hexene) (iPPH)-supported catalysts. These materials can easily be transformed into many different functional groups that are useful for synthesis and catalysis using post-polymerization functionalizations. In the course of our work, we became interested in exploring other chemistries, in addition to the work previously reported.
Typically, ROMP-based polymers’ properties can be tuned through the inclusion of various functionalities using either pre-or postpolymerization functionalization reactions. Many of these reactions require transition metal catalysts or excessive amounts of organic solvents, rendering them not sufficiently "green". We have developed and utilized various, metal-free, click-type processes for the construction and functionalization of ROMP monomers and polymers.
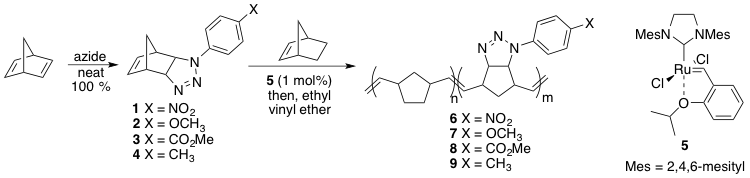
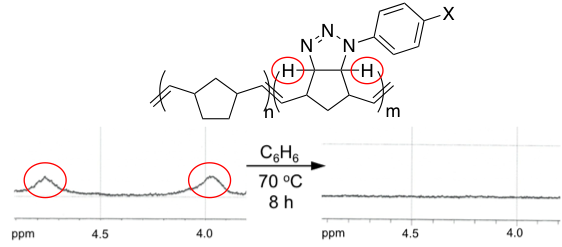
The first example of this work is based off of an old azide-norbornadiene cycloaddition (Scheme 1). Various aromatic azides (1-4) could be subjected to a reaction with excess norbornadiene under solvent free reaction conditions to form a series of triazolines in good yield. Each of these derivatives could be subjected to copolymerizations in the presence of Hoveyda-Grubbs 2nd generation initiator 5 to provide copolymers 6-9. We are also able to show that the resulting polymeric triazolines could undergo a smooth loss of nitrogen (N2) to be converted into aziridines upon heating, which can easily be monitored by 1H NMR (Figure 1).
Scheme 1. Metal-free triazoline formation and subsequent ROMP with initiator 5.
Figure 1. Partial 1H NMR spectrum showing loss of N2
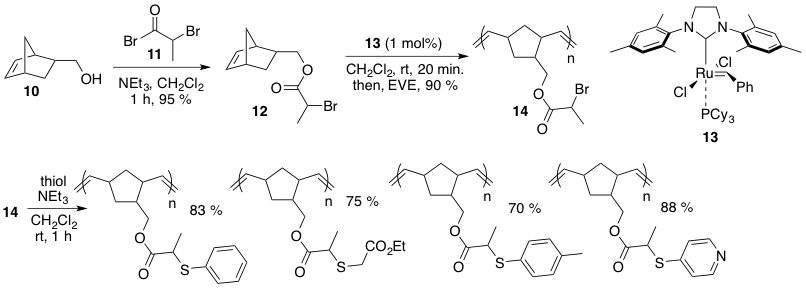
We have also become interested in investigating other metal-free click reactions for the post-polymerization functionalization of ROMP-derived materials. In this light, we found that a facile nucleophilic substation reaction between thiols and a-bromo esters (thio-bromo click) could efficiently be used for this purpose. This work involved the preparation of a-bromo ester-decorated polynorbornenes followed by their functionalization with various thiols (Scheme 2).
Scheme 2. Preparation of a-bromo ester 12, its ROMP, and thio-bromo click reactions.
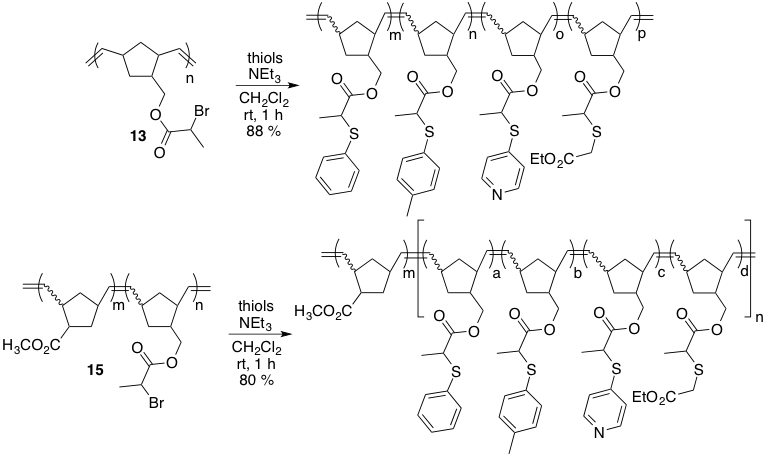
We were also able to show that this reaction could be carried out in a one-pot fashion for the installation of various functional groups in one step. This could be efficiently performed on homopolymer 14 as well as block copolymer 15.
Scheme 3. One-pot thio-bromo functionalizations of 13 and 15.
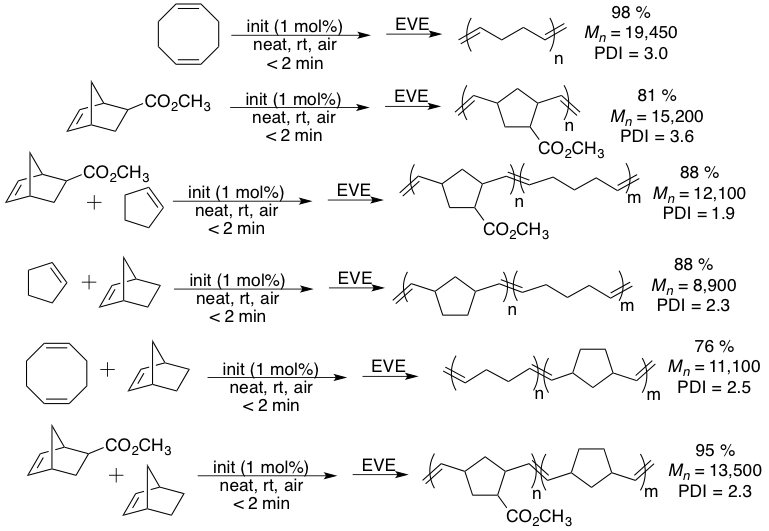
In addition to exploring green methods for polymer functionalization, we are also interested in looking at greener methods for polymer synthesis. In this respect, we developed a simple, solvent-free, bench top method for the preparation of ROMP-derived materials. This is beneficial as most ROMP reactions are carried out using sophisticated apparatus (glove box, Schlenk line) designed to provide inert atmospheres in which to carry out the reactions. Furthermore, these materials are almost always obtained using wasteful solvent precipitations. We developed a method that almost completely eliminates solvent waste from ROMP reactions. Furthermore, this can be run in an open flask on the bench top (Figure 2). This method could be used to provide homopolymers as well as copolymers and was not completely restricted to liquid monomers (Scheme 4). Various ROMP monomers could be polymerized and could be easily obtained after passing reaction through alumina to remove the residual catalyst. The materials could be analyzed by NMR as well as gel permeation chromatography (GPC).
Figure 2. Cartoon depicting this simple, solvent free ROMP process.
Scheme 4. Solvent-free ROMP reactions.