Reports: DNI1052830-DNI10: Synthesis and Electrochemical Characterization of Novel Polyanion Materials for High Capacity Li-Ion Cathodes
Candace K. Chan, PhD, Arizona State University
Overview
High capacity and high rate lithium-ion batteries (LIBs) with low cost and improved safety characteristics constitute a major requirement for electric vehicles, portable electronics, and other energy storage applications. Recently, there has been a great deal of interest in polyanion [(XOn)m-] materials for cathodes due to their low cost, good stability, and safety. An attractive feature of polyanions is that their reaction potential can be tuned through the ionic character of the anion. Additionally, many polyanions exist naturally as minerals or are formed as industrial waste or corrosion products. Therefore, they may represent a low-cost and abundant source of energy storage materials. The goal of this research project is to synthesize and investigate the electrochemical properties of novel polyanion electrode materials.
In this project, the following mixed oxo-anion materials were investigated: 1) the layered mineral brochantite, Cu4(OH)6SO4, one of the constituents of the green patina on the State of Liberty; 2) compounds based on jarosite, AM3(OH)6(SO4)2 , A = Na or K, M = Fe or V, which are widely used in the metallurgical industry to precipitate iron from acidic processing solutions; and 3) a novel titanium phospho-sulfate H1-xTi2(PO4)3-x(SO4)x with NASICON structure.
Significant Results
(1) Brochantite, Cu4(OH)6SO4
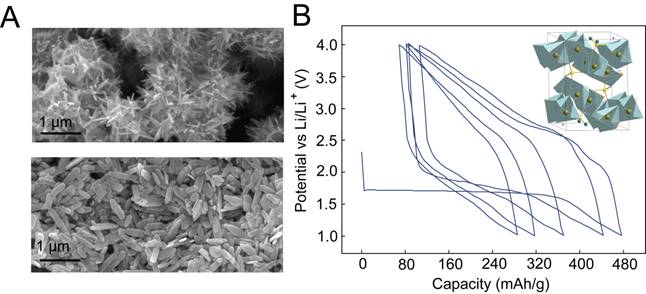
Nanostructured brochantite was prepared using microwave-assisted hydrothermal synthesis (Fig. 1A). Brochantite nanoplates were shown to demonstrate the full theoretical discharge capacity of 474 mAh/g upon reaction with lithium, with the materials following a conversion reaction mechanism corresponding to the 2 electron reduction of copper. The brochantite structure was investigated using X-ray diffraction (XRD), electron microscopy, and X-ray photoelectron spectroscopy (XPS) to understand the structural transformations after electrochemical cycling. XRD characterization suggested that the discharge products consist of Cu nanoparticles too small to be detected by X-rays within an amorphous matrix. High Coulombic efficiencies indicate that the conversion reaction in brochantite nanoplates has high reversibility, unlike other Cu conversion materials such as CuF2. Owing to the formation of small Cu nanoparticles during the discharge of brochantite nanoplates, good reversibility was observed, but long-term capacity retention was limited by Cu dissolution into the electrolyte during charging. The results indicate that copper hydroxysulfate materials such as brochantite may be promising electrode materials for lithium-ion batteries if this dissolution problem is addressed.
Figure 1. (A) Scanning electron microscopy images of brochantite nanoplates prepared using hydrothermal synthesis. (B) Galvanostatic cycling of brochantite nanoplates using C/20 rate. Inset shows the crystal structure of brochantite.
(2) Jarosite compounds, AM3(OH)6(SO4)2, M = Na or K; B = Fe or V
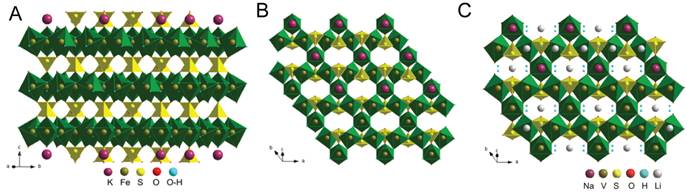
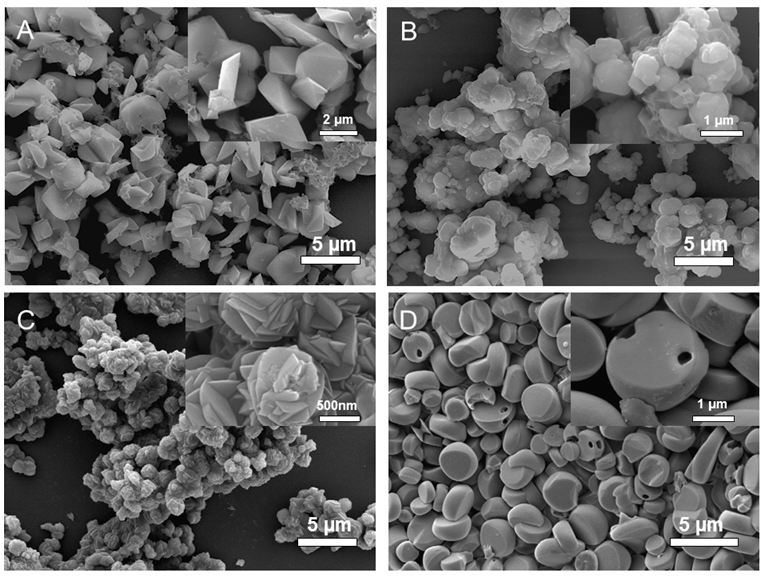
Jarosite compounds contain layered structures with open channels that may facilitate the electrochemical insertion of Li (Fig. 2). Microstructured jarosite materials were prepared using microwave-assisted hydrothermal synthesis to obtain particles with more uniform morphology than conventional hydrothermal reaction. From the results shown in Figure 3, we can see that the composition plays an important role in the morphological variation of the jarosite samples. However, the morphology within each type of jarosite was fairly uniform using microwave hydrothermal reaction.
Figure 2. Crystal structure of jarosite KFe3(OH)6(SO4)2 (A) showing the layers along the c-axis and (B) vacant channels along the [-1-21] direction. (C) NaV3(OH)6(SO4)2 with Li in the 9e sites within the channels.
Figure 3. Scanning electron microscopy images of jarosite made using microwave-assisted hydrothermal method (A) Na,Fe-jarosite, (B) K,Fe-jarosite, (C) Na,V-jarosite, (D) K,V-jarosite.
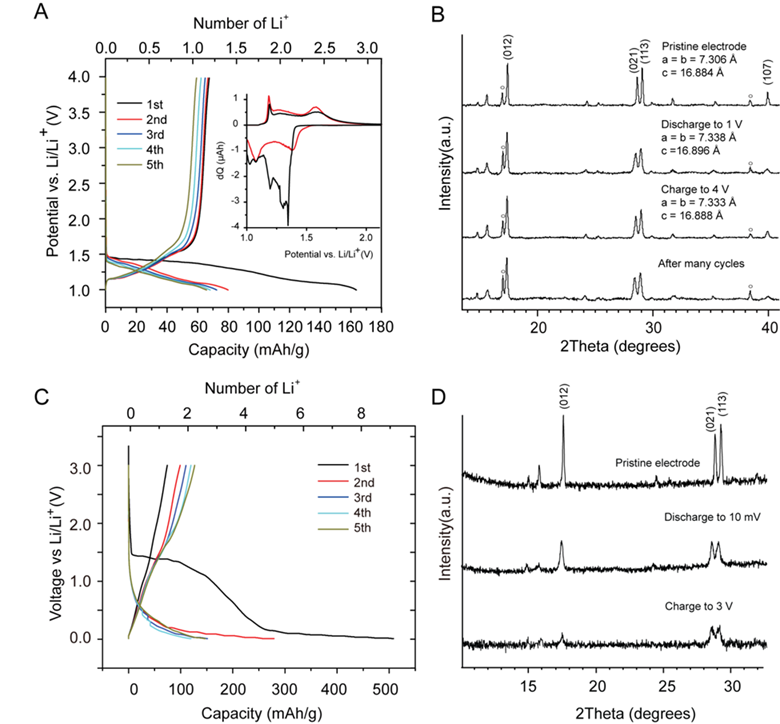
The electrochemical properties of the materials were also composition dependent. Furthermore, the reaction potential determined the lithiation mechanism. At potentials >1.5 V vs. Li/Li+, a reversible insertion-type reaction was observed in Na,Fe-jarosite but not in K,Fe-jarosite due to the substitution of some K+ for H3O+, which decreased the interlayer spacing and inhibited Li+ insertion. Reversible insertion-type reactions were observed in both vanadium jarosite analogs, and XRD characterization of the lithiated compound indicated Li+ may be occupying the space in the empty channels, as shown in Figure 2c. Below 1 V vs. Li/Li+, all four jarosite compounds studied displayed high capacities corresponding to the 3 electron reduction of Fe3+ or V3+. XRD characterization showed the crystallinity of the samples decreased after lithiation, which is consistent with a conversion-type reaction. Figure 4 shows the voltage profile and XRD data of NaV3(OH)6(SO4)2 as an example.
Figure 4. (A) Voltage profiles of Na,V-jarosite discharged/charged between 1 – 4 V vs. Li/Li+, inset shows differential charge plot for first 2 cycles; (B) XRD after cycling between 1 – 4 V vs. Li/Li+. (C) Voltage profile of Na,V-jarosite discharged/charged between 10 mV – 3 V vs. Li/Li+. (D) XRD after cycling between 10 mV – 3 V vs. Li/Li+.
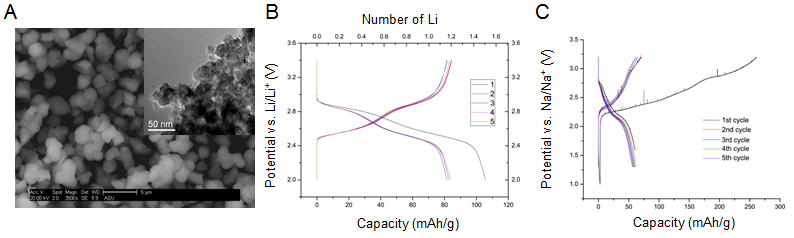
(3) NASICON-structured titanium phospho-sulfate, H1-x Ti2(PO4)3-x(SO4)x
NASCION structures are framework compounds of the form M2(XO4)3 where the tetrahedral oxo-anions and metal cation octahedral form a framework of “lantern units” with ion channels connected in three-dimensions, which can host cations. These materials have been studied in the past as Li-ion battery electrodes. A new titanium-based compound with mixed phosphate and sulfate anions, H1-x Ti2(PO4)3-x(SO4)x (HTPS), was recently synthesized by our collaborator Prof. Don Seo at Arizona State University (Fig. 5A). The electrochemical properties of HTPS were evaluated in Li and Na cells for the first time. As shown by the voltage profiles in Figure 5B-C, both Li and Na ions could be reversibly inserted into HTPS electrochemically.
Figure 5. (A) Scanning and transmission (inset) electron images of HTPS; Voltage profiles of HTPS in (B) Li and (C) Na half-cells.
Impact
These studies show the opportunity for using natural minerals in lithium and sodium-ion batteries and lay the groundwork for further investigation into other similar materials. Due to the diverse and rich variety of structures in the mineral world, we hope that this can lead to the identification of other promising electrode materials for electrochemical energy storage applications.
This funding has been valuable for the PI as a young investigator and greatly helped her build up her lab by supporting travel and student training. The project trained one graduate student and undergraduate in synthesis, structural characterization techniques, and electrochemical testing protocols. The work resulted in several journal articles, two patent applications, and several presentations at national conferences.