Reports: UNI351964-UNI3: Utilizing Carboxylic Acids to Promote Rapid and Selective Iron Catalyzed Epoxidations of Alkenes
Christopher Goh, PhD, AM, BSc, Williams College
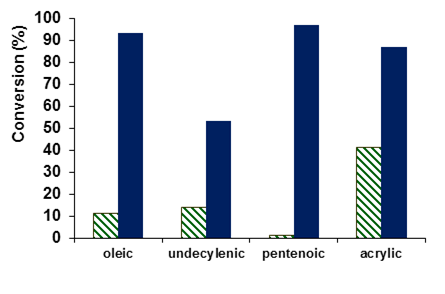
During the first half of the grant period, we successfully completed the research objective of the grant proposal to examine the influence of the presence of a carboxylic acid functional group in alkenes on the rate and selectivity of the epoxidation of alkenes. We confirmed our hypothesis about the positive effect of the carboxylic acid functional group on yields and selectivity of epoxide formation. We focused on using the known epoxidation catalyst [(bpmen)Fe(OTf)2] (bpmen = N,N’-dimethyl-N,N’-bis(2-pyridylmethyl)-1,2-diaminoethane) to oxidize the substrates oleic acid, undecylenic acid, 5-hexenoic acid, 4-pentenoic acid and acrylic acid, with hydrogen peroxide as oxidant. Comparisons with the analogous ester substrates demonstrated the beneficial impact of the acid functional group on conversion (Figure 1).
Figure 1: Comparison of the conversion of ester (green, lined) and corresponding acid substrates (blue, solid) demonstrating the positive impact of the acid functional group on conversion.
For oleic, undecylenic and acrylic acids, epoxide product formed with moderate to high conversions and high selectivities. 4-pentenoic acid and 5-hexenoic acid were oxidized to lactones, most likely via epoxide intermediates.
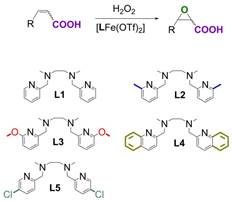
To probe the impact of ligand structure on catalysis, we synthesized 4 derivatives of the iron-bpmen complex with varying substitutions on the pyridine rings (Figure 2).
Figure 2: Iron complexes synthesized to probe the impact of changes at the pyridine position on oxidation catalysis.
Single crystal analyses showed the impact of these variations on solid state structures. The iron centers possessed distorted octahedral geometries, owing to the steric requirements of the tetradentate ligands. The two pyridine donor moieties were trans to each, and the ligands adopted the cis-a conformation typical of iron complexes with bpmen-type ligands. Of the derivative complexes, only the N,N’-dimethyl-N,N’-bis(5-chloropyridin-2-ylmethyl)-1,2-diaminoethane variant showed appreciable catalytic activity. Increasing the steric bulk of the ligand in the ortho position of the pyridine rings appeared to significantly reduce oxidation activity.
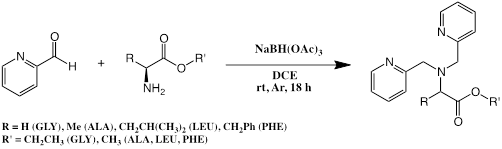
Having accomplished the first research objective, we worked on the proposal’s second objective of incorporating carboxylic acid functional groups into the ligand framework. Our first targets were the iron complexes of the tripodal ligands in Figure 3. We were able to synthesize alkyl ester variants of these ligands from the corresponding esters of glycine, alanine, leucine and phenylalanine.
Figure 3: Ligand framework containing a carboxylic acid for oxidation catalysts.
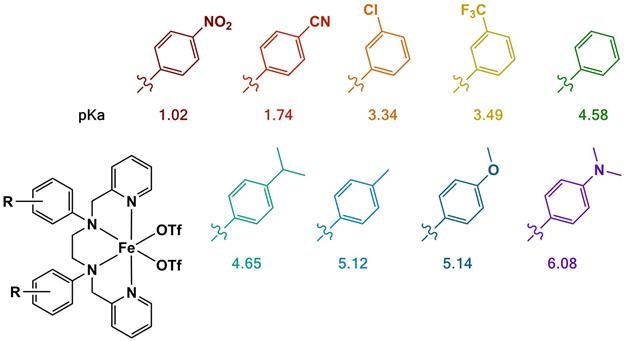
As anticipated, iron complexes of these ester ligands did not show appreciable activity catalyzing the oxidation of the test alkenes cyclohexene and styrene. The syntheses of the carboxylate analogs presented us with significant synthetic challenges, most likely owing to the amphoteric nature of the ligand targets. To provide students with a positive research experience, we redirected our synthetic efforts to modifying the amine position of the parent bpmen framework to include a range of substituted anilines (Figure 4). The substituents chosen permit investigating the impact of electronic variations without significantly altering the steric nature of the ligands within the family of aromatic amines. Initial results showed that the aniline derivatives have similar efficiencies in oxidizing undecylenic acid and reduced activities for oxidizing cyclooctene compared to the original bpmen-complex. This research effort continues, and once the current list of ligand targets is completed, we will target a benzoic acid variant of this ligand family.
Figure 4: Target iron complexes to probe the impact of changing the ligand framework at the amine position. The pKa values shown represent literature values of parent anilines.
In another exploratory senior thesis project, we polymerized epoxides of fatty esters generated by the oxidation of fatty acids and esters. Acrylation of the epoxide moiety and controlled radical polymerizations of the acrylated fatty ester monomers led to low molecular weight products, with degrees of polymerization of about 10, regardless of condition of polymerization. We decided not to pursue this avenue further.
The impact of this PRF-funded research project has been multifold. The grant supported the research of 10 undergraduate students during a 3-yar period. Of these 10 students, 6 were first-generation students and 5 were from underrepresented minorities. By many accounts, the research experiences have been impactful for the students, and have played important roles in their decisions to pursue a career in research or medicine or to explore careers in teaching science. 3 students completed senior theses research on the funded project. Of these students, Lilli Morris and Claire Lidston are pursuing graduate degrees in chemistry at Cornell and Harvard University, respectively. Thanks to the funds provided by PRF, both students were able to present their work at ACS National Conferences in New Orleans and Denver, respectively. The third student, Areli Valencia taught high school science in Chicago for a year, before moving to a research associate position at NIH. Michael Girouard completed a semester-long senior independent research study (Fall 2012). After graduation, he spent a year as a clinical research coordinator before enrolling at Harvard Medical School this fall. Jeff Brewington (Summer 2013; other funding) graduated in 2014 and teaches in Oklahoma for Teach-for-America. Tamuka Chidanguro (funded summer 2013) and Jake Huerfano (January term 2014) completed senior thesis research in my laboratory, albeit on different projects. Tamuka is now a graduate student in polymer science at the University of Southern Mississippi, and Jake is applying to graduate school in chemistry, spending a gap year teaching. Matt Davies (summer 2014, other funding; year-long work-study student 2014-15) continued to explore his interest in research by joining a research group at the University of Toronto for the summer 2015. Jose Lopez, a current junior, is hoping to become a science educator after graduation – “I want to be the next Bill Nye” is his stated aspiration.
The PRF grant has supported our group’s continued efforts of synthesizing classes of closely-related ligands in gram quantities and storing these ligands for future use in other homogeneous catalysis projects, thus contributing to the research infrastructure of my laboratory. We continue working on iron-based oxidation catalysts, with one senior thesis student exploring modifications of the amine position on the ligand framework, and I look forward to many years of engaging our students in these research endeavors.