Reports: ND454436-ND4: Concerted Excited-State Hydride Transfer from Organic Models
Ksenija D. Glusac, Bowling Green State University
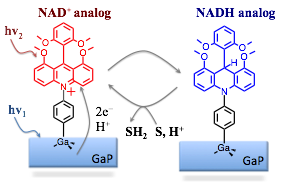
Introduction: Hydride transfer is a fundamental process that appears in many biological and chemical reactions. The aim of this project is to develop NADH-analog hydrides that can be generated using light, as presented in Figure 1. The photogenerated hydrides can then serve as alternative green catalysts for reduction processes (reduction of substrate S to SH2, Figure 1) used in synthesis of industrially relevant chemicals.
Figure 1. A schematic representation of proposed photohydrides catalysts using NADH/NAD+ analogs.
To develop the desired photocatalyst, three projects are currently underway in the Glusac labs: (i) Dye sensitization: The goal of this project is to find ideal conditions for efficient photoinduced electron transfer from excited NAD+ analog dye to the GaP; (ii) Two-for-one photoreduction: The current doubling mechanism (two-electron photosensitization) will be used to convert the NAD+ analog dye to the NADH analog; (iii) NADH analogs for proton reduction: The NADH-analogs will serve as reducing agents for the reduction of relevant substrates. The aim of this project is to study the structural effects that increase the hydricities of model NADH analogs and reduce the barrier for the hydride transfer reaction to hydride acceptors.
Impact: The research performed in this project is likely to lead to the discovery on environmentally-friendly photocatalysts for reduction of industrially-relevant chemicals. The financial support from the ACS PRF is enabling the Glusac group to obtain the preliminary proof-of-principle results of such photocatalysis. In addition, the PRF funds are providing the financial support for training of two PhD students, one working on the syntheses of model NADH/NAD+ analog compounds and the other working on the studies of interfacial electron transfer using time-resolved laser spectroscopy.
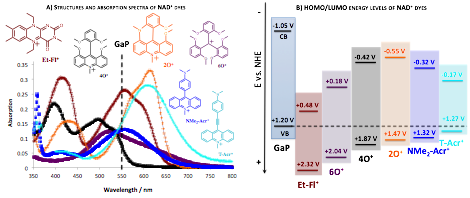
Research Summary: A series of NAD+-analog dyes were synthesized and their absorption spectra are presented in Figure 2. The HOMO-LUMO energies and absorption wavelengths of the model compounds indicate that the dye-sensitized hole injection to GaP is thermodynamically feasible.
Figure 2. Preliminary study of NAD+ analog dyes: A) structures and UV/Vis absorption spectra of selected dyes; B) energy levels of NAD+ dyes relative to the band energies of GaP. The electron injection from excited dyes to the valence band of GaP is thermodynamically feasible.
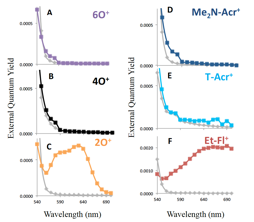
The photoelectrochemical response was tested using p-GaP(111)A photoelectrodes biased at -0.6 V vs. Ag/AgCl and immersed in deaerated1M KCl (aq) with and without the model compound dyes (Figure 3). In the absence of model dyes, the photoresponse below 550 nm arises due to the excitation of GaP electrode itself. In the presence of dyes, the sub-bandgap response was expected to occur at wavelengths above 550 nm due to sensitization. Surprisingly, four dyes (6O+, 4O+, Me2N-Acr+ and T-Acr+) did not exhibit significantly different photocurrents as compared to the blank solution, especially at wavelengths where the respective absorption of dyes was the strongest. In contrast, the 2O+ and Et-Fl+ dyes elicited substantially higher photocurrents than the blank electrolyte.
Figure 3. External quantum yield measurements of etched p-GaP(111)A sensitized with cationic dyes (<50 μM) in nitrogen-purged 1 M KCl (colored squares) or without dye (gray diamonds).
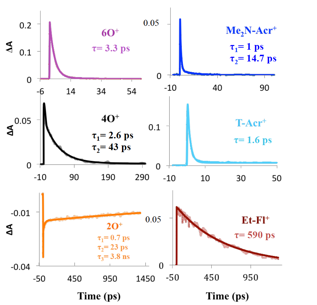
The sensitization results described above showed that only two dyes (Et-Fl+ and 2O+) exhibited efficient charge injection into GaP, despite the fact that the thermodynamics analysis predicted efficient injection from all model compounds. One possible explanation for such behavior was that most of the dyes exhibited short-lived excited-states and that the internal conversion to the ground state efficiently competed with the charge injection into p-GaP. To investigate the excited-state behavior of the model dyes, femtosecond pump-probe measurements were performed for dyes in solution (Figure 4). The results of these time-resolved studies indeed show that the only two dyes that exhibit longer lifetimes (2O+ and Et-Fl+) were exactly the dyes that were found to efficiently sensitize GaP. These results confirmed that the charge injection from the “inefficient” dyes to GaP did not compete with the internal conversion, indicating that the rate of charge injection from these dyes to GaP did not occur on the sub-picosecond timescale under the employed conditions.
Figure 4. Kinetic traces for model compounds in acetonitrile upon excitation at: λexc = 400 nm for Et-Fl+; λexc= 615 nm for 2O+; λexc= 525 nm for 6O+; λexc= 565 nm for Me2N-Acr+; λexc= 525 nm for 4O+; and λexc= 600 nm for T-Acr+.
Future Work: The work presented above demonstrates that the photoreduction of NAD+ analogs can be achieved using semiconductors with appropriate valence band energies. The future work will focus on the studies of hydricities of the model NADH analogs derived from the cations presented in Figure 2.