Reports: UR552145-UR5: Synthesis of Ruthenium Dioxide Nanoparticles and Clusters with Tailored Size-and Surface-Dependent Properties for Enhanced Catalytic Applications
Vladimir Kitaev, Wilfrid Laurier University
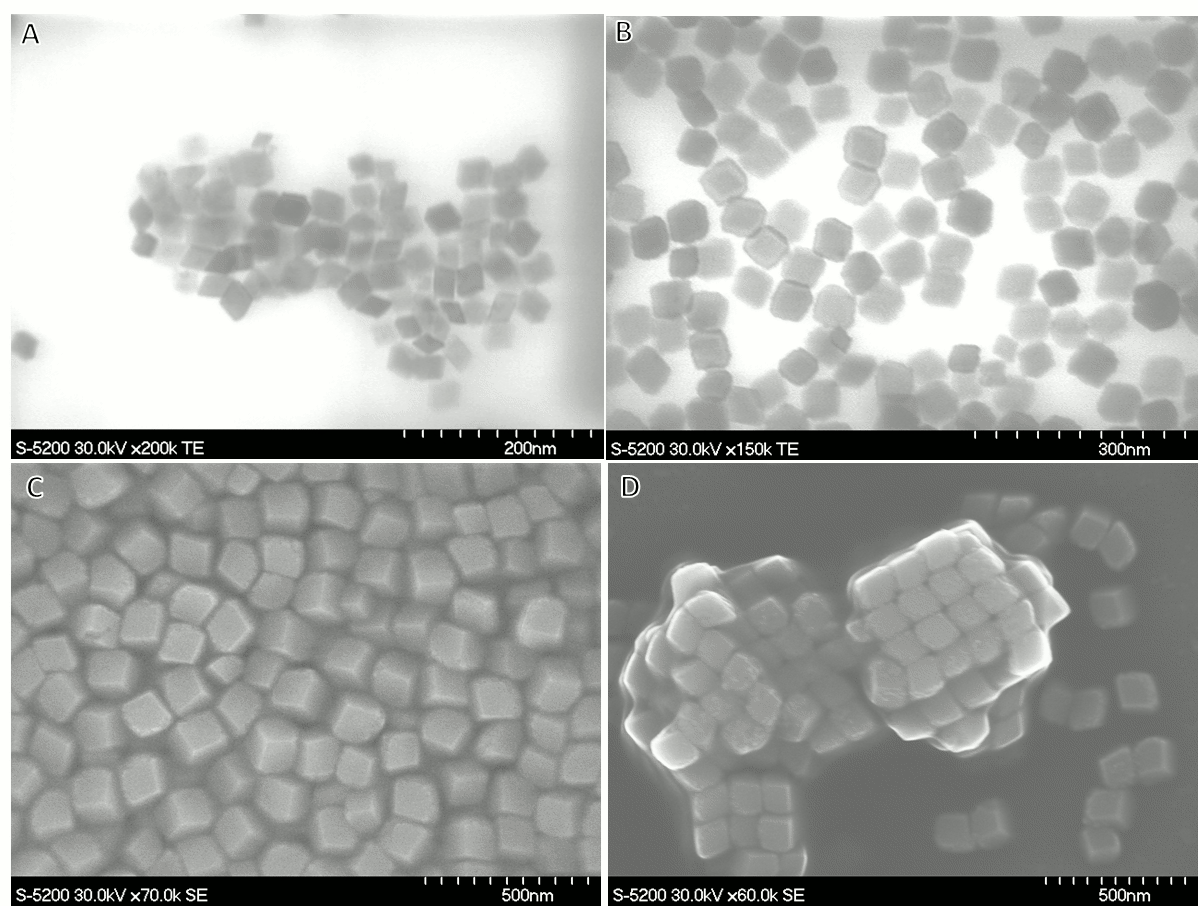
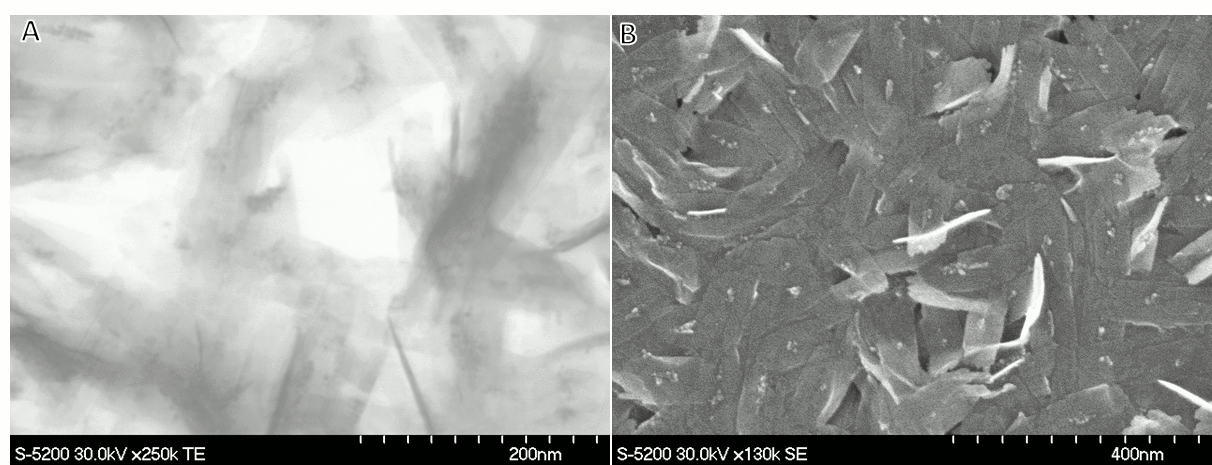
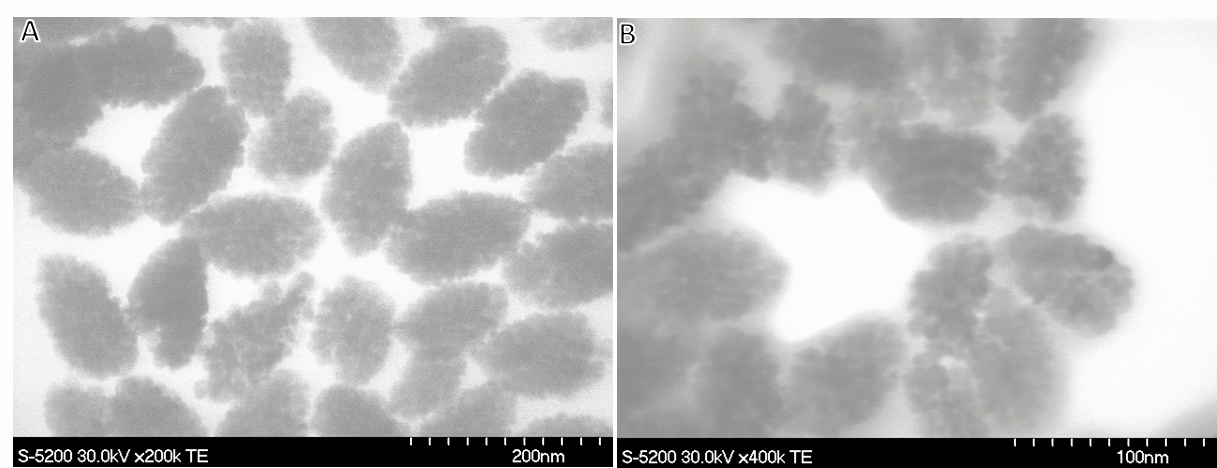
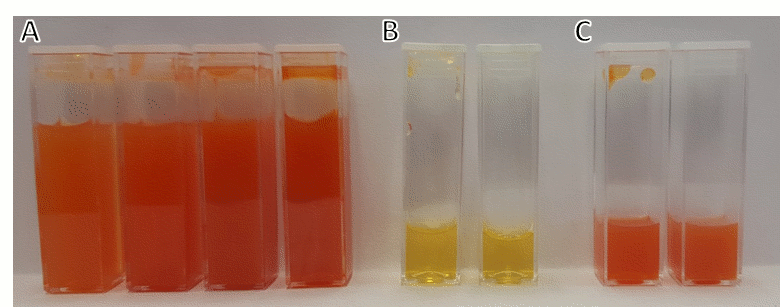
Vladimir Kitaev, Wilfrid Laurier University




Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society