Reports: ND752970-ND7: Dynamics of Confined Polymer Liquids and Glasses under Positive and Negative Pressures
Rodney D. Priestley, PhD, Princeton University
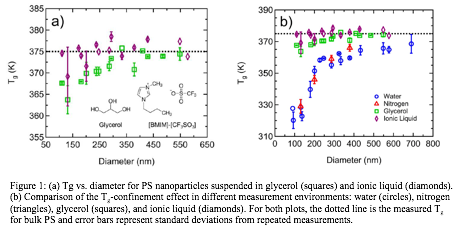
Rodney D. Priestley, PhD, Princeton University

Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society