Reports: ND552921-ND5: Tailoring Surface Electronic Structure: Probing the Effect on Chemical Activity
Regina Ragan, University of California (Irvine)
Hybrid nanosystems, composed of metal-carbon interfaces, are being developed for energy applications including fuel cells and supercapacitors. Graphene has a large effective surface area, stable surface chemistry, and excellent electronic and mechanical properties, properties highly desirable for energy storage systems. Graphene-hybrid composites have been considered for improving supercapacitor energy density by increasing specific active surface area, enhancing the conductivity of electrode surfaces, or incorporating Faradaic reactions. Recent work on single layer graphene in the presence of surfactants and other surface moieties has shown higher capacitance than both graphene oxide and pristine graphene. These findings suggest exciting opportunities using doped or metal decorated graphene interfaces to alter surface electronic structure. Most work on carbon-based systems has been limited to characterizing performance of 2D surfaces or random 3D networks, due to the difficulty of synthesizing mechanically and electronically robust constructs with well-organized internal microstructure. Thus, during the funding period, a method for the synthesis of porous 3D graphene-hybrid composites with uniform, connected, and tunable internal pore morphology was developed alongside methods for coating these structures with Pt nanoparticles. The intrinsic surface electronic properties of graphene-based interfaces with nitrogen dopants and defects, that have been shown to increase oxygen reduction activity, was also investigated to provide needed fundamental understanding of the design of 3D graphene-hybrid composites for enhanced electrochemical performance.
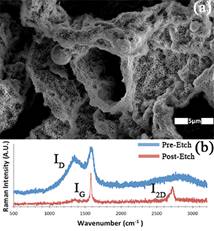
3D templates were fabricated using bicontinuous interfacially jammed emulsion gel (bijels) templates. Bijels form through arrested spinodal decomposition in a mixture of partially miscible fluids and neutrally wetting colloidal particles that irreversibly adhere to the fluid-fluid interface, and become jammed when the interfacial area is sufficiently reduced (due to phase separation). The result is a mechanically stable, gel-like emulsion with the unique tubular microstructure, which is characterized by regular co-continuous domains with uniform size and a large interfacial area. The resultant bijel-derived template, made of polyhexanediol diacrylate, was immersed in a saturated solution of PdCl in ethanol to activate the polymer followed by a NiCl solution for electroless plating of pure nickel on internal surfaces. The coated scaffold was pyrolyzed at 500°C under an H2/Ar flow (75 SCCM), where C diffuses into Ni. C segregates to the surface and forms graphene during cool down. Raman Spectroscopy was performed before and after etching Ni. A disordered peak, ID, (blue curve in Figure 1b) is observed at 1350 cm-1, alongside a 1580 cm-1 peak, IG, that is the in-plane vibration of the C-C bond. After etching Ni in aqua regia, and Raman spectroscopy was performed again (red curve in Figure 1b) showing the characteristic 2D peak (2690 cm-1), I2D, which is the second-order overtone of in-plane vibrations. The I2D/IG ratio indicates few-layer graphene. Scanning electron microscopy images (Figure 1a) showed a mechanically stable structure composed of a nanoporous carbon shell with the overall spinodal-like morphology of bijel templates (with domains ~2.5 mm, and smaller pores, ~280 nm). Electron Dispersive Energy Spectroscopy also showed a large C and a weak Ni signal, indicating that the structure is primarily composed of carbon.
Figure 1: (a) SEM image of 3D graphene structure fabricated with a bijel scaffold. (b) Raman spectra before (blue) and after (red) etching Ni.
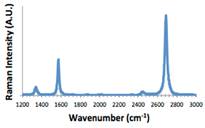
Ni:Cu alloys of varying compositions were investigated on 2D surfaces in order to control the number of graphene layers with C solubility. Figure 2 shows Raman spectra of graphene after annealing SU8-2002 (polymer carbon source) under a reducing atmosphere (4%H2 /96%Ar) and after etching Ni:Cu with FeCl3. The results show high quality graphene film with I2D/IG ratio of ~2.29, characteristic of single layer of graphene on Ni:Cu when the alloy composition is 5:95. Additionally, electrochemical deposition methods of Pt nanoparticles. Electrochemical methods are available to high surface area structures thus it was important to determine feasibility on graphene surfaces. Figure 3 shows an Atomic Force Microscopy (AFM) image of Pt nanoparticles electrodeposited on a graphene surface.
Figure 2: Raman spectrum of 5:95 Ni:Cu sample with SU-8 2002 on the surface after a 1000 C anneal and etching with FeCl3.
Figure 3: AFM image (scale bar in nm) of graphene surface after electro-deposition of Pt in 4.8 mM H2Pt Cl6 solution
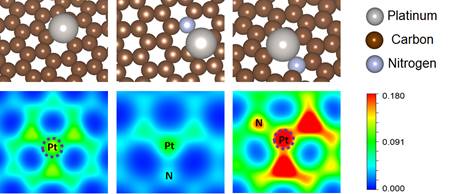
Ab initio calculations of hybrid graphene surfaces were also performed to elucidate how atomic and electronic structure is influenced by this nanoenvironment. Density Functional Theory (DFT) calculations were performed using the local-density approximation (LDA) psuedopotential, which correlate well with experimental results for graphene.107 Investigations of Pt atom interactions on a single vacancy on undoped and N-doped graphene are shown in Figure 4. The relaxed structures (top row) vary with nanoenvironment of Pt. On undoped surfaces, a Pt adatom will insert into a vacant site. When a nitrogen dopant sits in a vacancy, the charge density plot (bottom row) shows that the N facilitates interaction of Pt with neighboring C, resulting in charge depletion from its topmost. The first two results confirm previously reported DFT calculations. However, scanning tunneling microscopy data often observes pyridinic N-doping in graphene, not previously studied with DFT, where a N atom substitutes C in a divacancy defect. Pt is again strongly attracted to the vacancy site and N fills the other empty sites to reconstruct to approximate the hexagonal graphene lattice. The charge density plot is significantly different and this may elucidate the origin of enhanced ORR activity of these structure.
Figure 4: Relaxed structures of Pt atom on single vacancy on undoped graphene (left), N-doped graphene (middle), and pyridinic N-doped graphene (right). (below) Corresponding harge density plots along the (110) plane.
These findings during the New Directions funding period are important results to show the formation of porous, 3D graphene structures in a morphology relevant to energy storage systems. Furthermore, the developed fabrication methods are compatible with doping graphene with nitrogen and sulfur during pyrolysis by adding NH3, H2S into the reducing gas environment to control electronic structure. First principles calculations provide insight on how to process materials to improve electrochemical performance.