Reports: ND453415-ND4: Mechanistic Studies of Transition-Metal Catalyzed, MAO-Assisted Olefin Polymerization
Rainer Ernst Glaser, Ph.D., MS, Dipl.-Chem., University of Missouri, Columbia
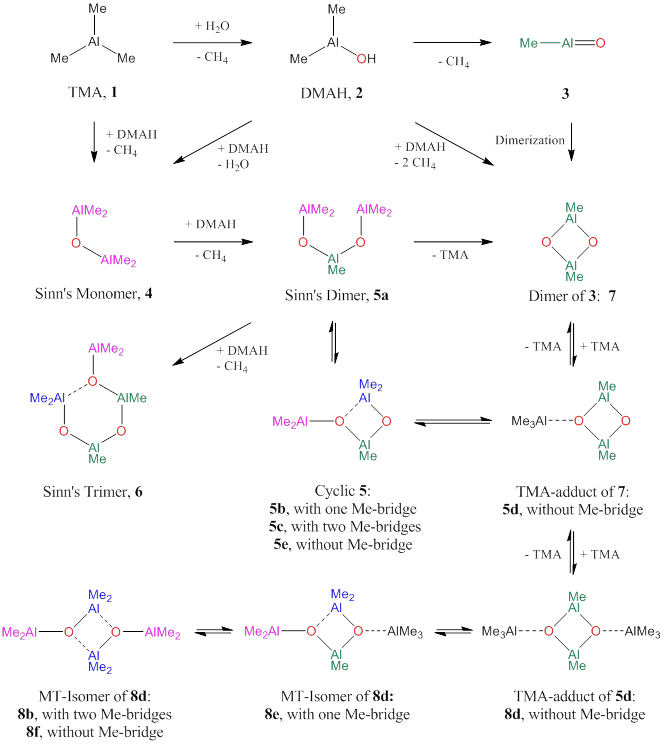
Even though methylaluminoxane (MAO) is a common co-catalyst in olefin polymerization, its structures and mechanistic functions remain unclear and a variety of species have been discussed. There is no doubt that hydrolysis of alkyl–Al bonds results in the formation of HO–Al bonds, the release of alkanes, and the formation of acyclic and cyclic systems with Al–O–Al bridges by further inter- and intramolecular alkane elimination. Initial ab initio studies of the hydrolysis of trimethylaluminum (J. Am. Chem. Soc. 2011, 133, 13323-13336) supported the possibility to form cyclodialuminoxane 7 from 2, and we have now performed a comprehensive study of the chemistry outlined in Figure 1.
Figure 1. Possible products formed by partial hydrolysis of trimethylaluminum 1: dimethyl-aluminum hydroxide 2, methylaluminum oxide 3, permethyldialuminoxane 4, permethyltrialuminoxane 5a, permethyltetraaluminoxane 6, and permethylcyclodialuminoxane 7. Methyl-transfer isomerization of 5a provides a path to cyclic 5 and the TMA adduct of 7 without going through 3. TMA addition to cyclic 5 and methyl-transfer provides a path to 8, the dimer of 4.
The reaction of water with TMA, 1 affords DMAH, 2 in an exothermic reaction (Scheme 1). Once formed, DMAH might undergo intramolecular CH4 elimination to form methylaluminum oxide 3, or react with TMA to 4 by intermolecular CH4 elimination, or condense with another DMAH molecule to form 4 via a Me2Al-O-AlMe-OH. As the concentration of 4 grows, DMAH has the additional option of intermolecular coupling with 4 and this path to the formation of 5 will become significant if the basicity of the methyl group in 4 exceeds those of TMA and DMAH. Another such coupling would afford 6. The formation of the linear polymer 6 is preferred to the branched Al(OAlMe2)3 isomer because the Lewis acidity of the AlMe2 moiety is higher than that of the AlMe group in 4 (i.e., O as a p-donor). We have now studied possible outcomes of the reaction of 5 with TMA instead of DMAH. We have shown that 5a can cyclize to methyl-bridged species 5b and 5c, and that methyl-transfer provides a path to 5d, the TMA adduct of 7, without going through 3 and without the need for the reactive dimerization of 2. We also have been able to show that TMA addition to cyclic isomers of 5 may lead to various isomers of 8 and these include 8b, which is formally the dimer of 4.
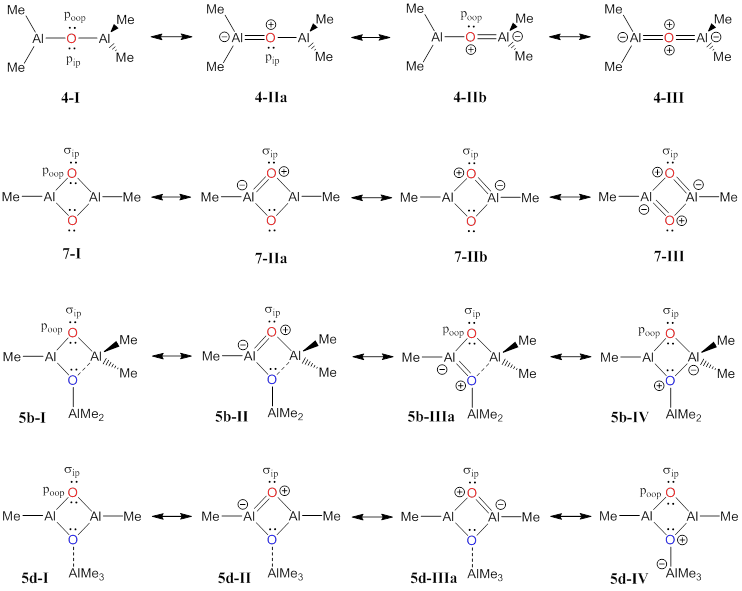
Figure 2. Differences in intramolecular O→Al p-dative bonding in acyclic and cyclic aluminoxanes, respectively, affect their capacity to serves as Lewis acceptors and Lewis donors in intermolecular interactions.
We have computed reaction energies for all of these reactions at electron-correlated levels of electronic structure theory. One of the key concepts of the present studies relates to the significant differences in the strengths of the Lewis acceptors and of the Lewis donors in acyclic and cyclic aluminoxanes, respectively, and this is exemplified in Figure 2 for the simplest cases of 4 and 7, respectively. The sp-hybridized oxygen in 4, a reverse-polarity heterocumulene, may employ two lone pairs (pip and poop) for p-dative bonding to two Al centers, while each oxygen in 7 is approximately sp2-hybridized and can employ just one lone pair (poop) for intramolecular p-dative bonding to two Al centers. We have shown that the structural space accessible to donor-stabilized trialuminoxane 5 includes cyclic structure of types 5b and 5d, and one oxygen in both of these structures is very similar to the oxygens in 7. Hence, one has every reason to expect that the oxygens in 7 and in cyclic 5 are much better Lewis bases in intermolecular interactions as compared to acyclic aluminoxanes, and the intermolecular interaction of special interest concerns the ligating ability of the MAO species to transition metal catalysts in olefin polymerizations.
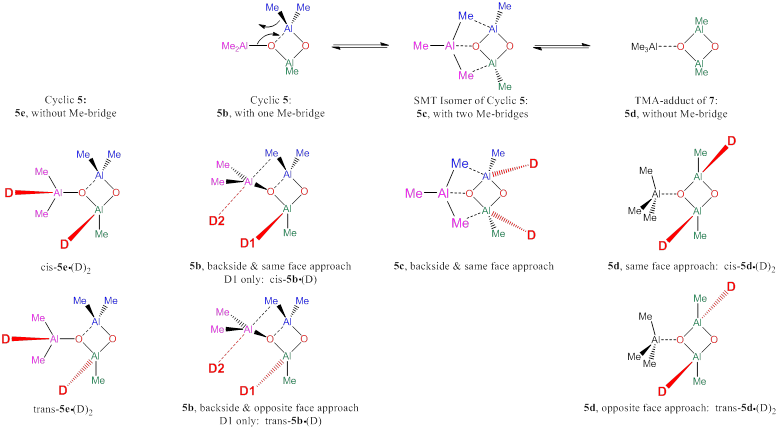
The aluminoxanes contain strong Lewis acceptors and Lewis donors and their chemistry is likely to be affected by solvation as well as aggregation, and two of these effects have been assessed. First, we have included dimethyl ether molecules to model the interaction of tri-coordinate Al sites by a representative ether O-donor. It is one of the key features of cyclic aluminoxanes that the O-atoms in these rings are far more nucleophilic than the O-atoms in open aluminoxanes. Hence, cyclic aluminoxanes are likely to aggregate with Lewis acids and we have explored the stabilization of the cyclic isomer of 5 by aggregation with TMA. The interplay of both donor and acceptor stabilization reveals a great malleability of the structures and stabilities of the aluminoxanes and the effects of donor stabilization are illustrated in Figure 3 for cyclic 5. The TOC graphic shows the optimized structures cis-5d•(OMe2)2 and cis-5e•(OMe2)2.
Figure 3. In the presence of donor stabilization, structures 5b and 5c are converted into cis- and trans-5b•(OMe2), cis- and trans-5d•(OMe2)2, and cis- and trans-5e•(OMe2)2.
International Collaboration in Research and Education. PRF funding enabled us to continue collaborations with Dr. W.-H. Sun of the Institute of Chemistry, Chinese Academy of Sciences, Beijing (ICCAS), Dr. C.-Y. Guo of the University of the Chinese Academy of Sciences, Beijing (UCAS), and Dr. B. Wu of the Department of Chemistry, Northwest University, Xi’an (NWU). The collaborations have included mutual visits by faculty and students, and Ms. K. Yang, a 2015 intern at MU from Xi’an, has recently started her doctoral studies at MU.
The PI spent about six weeks in China on two trips with additional support by guest professorships at NWU and XMU (Xiamen). The PI presented on Structural Chemistry and Thermochemistry of MAO Formation. Studies of Cycloaluminoxane Ligands and of the Aggregation of Acyclic Aluminoxanes at the CB2015 Conference, The Chemical Bonds at the 21st Century, Xiamen University (June 16, 2015), he presented a plenary lecture on Teaching Scientific Writing and Scientific Peer Review - Framework of an Assignment-Based Curriculum at the 1st International Conference on the Development of English Across the Curriculum, The Hong Kong Polytechnic University (Dec. 15, 2015), and he also presented numerous invited lectures in China and in the US.