Reports: UR353427-UR3: Homogeneous Fischer-Tropsch Catalysts for the Conversion of Syngas into Higher Order Hydrocarbons
John D. Gilbertson, PhD, Western Washington University
The predicted decrease in the availability of petroleum and petroleum-based products has sparked renewed interest into the conversion of synthesis gas (syngas) into chemical feedstocks and fuels. Conversion of syngas (CO + H2) to liquid hydrocarbons and a-olefins by the Fischer-Tropsch (F-T) reaction offers promise, but the low selectivity of these reactions by the heterogeneous route is a major drawback. The development of homogeneous systems for the F-T reaction has the potential to increase both selectivity and understanding of the F-T mechanism. Funded by an Undergraduate Research grant from the American Chemical Society Petroleum Research Fund (UR, June 2013 – August 2016), metal-ligand complexes composed of pendant Lewis/Bronsted acids/bases and redox-active sites within the ligand scaffold are being investigated to study the C-H and C-C bond forming steps in the F-T reactions of CO.
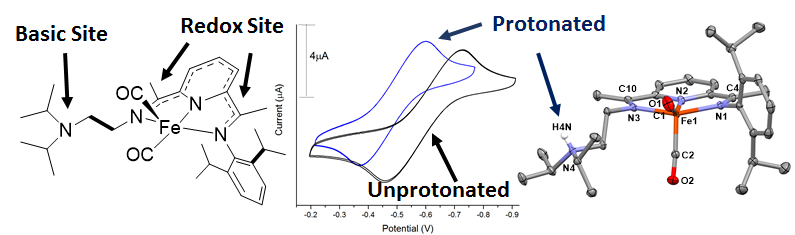
Progress since the last report includes the publication of an in-depth study of the relationship between protonation state of the secondary coordination sphere pendant base and the redox-activity of the PDI ligand scaffold (Inorg. Chem. 2015, 54, 7239–7248). Given the intimate nature of secondary coordination sphere protonation state and redox potentials in catalytic systems, we also reported the pH dependence of the potentials of the redox-active ligand in the reduced FePDI complexes. The protonated didpa ligand is capable of forming metal halogen hydrogen bonds (MHHBs) in neutral complexes and the solution behavior of the MHHBs was probed via pKa measurements and 1H NMR titrations of H-bond accepting strength. The relationship between the protonation state and the ligand-based redox activity was also probed utilizing where the reduction potential of the didpa scaffold was found to shift by 105 mV upon protonation of the reduced ligand in Fe(didpa)(CO)2.
Figure 1. Image outlining the relationship between the redox potential of the reduced Fe(didpa)(CO)2 complex and the protonation state of the ligand. The reduction potential of the complex shifts by ~100 mV upon protonation/deprotonation of the ligand.
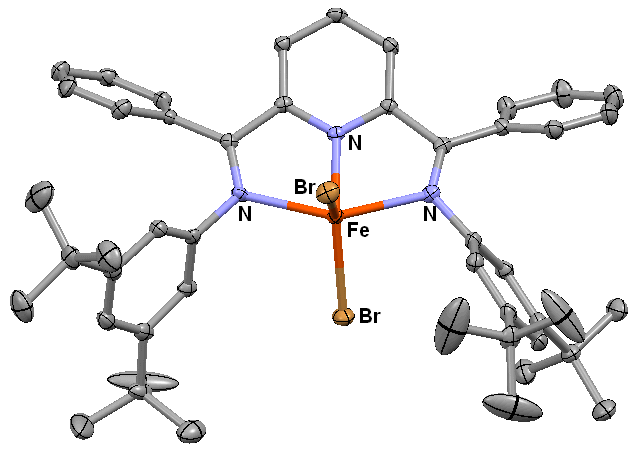
We are continuing to make progress on the synthesis of ligands that do not have protons capable of reacting with reductants such as triethylborohydride. We are attempting to eliminate any –CH3 and/or (CH3)2CH- groups in the ligand scaffold as one of the major goals outlined in the original proposal was to utilize the secondary coordination sphere to stabilize the formation of CO-derived iron formyls (formed from the addition of hydride to the reduced dicarbonyl species). However, reduced dicarbonyl species formed from the complexes similar to those shown in Figure 1 do not display any formyl formation upon the addition of hydride sources. Instead, deprotonation of the –CH3 groups on the 2,6-diacetylpyrindine core occurs upon the addition of hydride due to the acidity of the acetyl protons in these complexes. As can be seen in Figure 2, the t-butyl benzoyl analog has been successfully synthesized and characterized. This compound is also soluble in ether which has allowed us to study hydride transfer reactions from hydride sources such as [Ni(DMPE)2H][PF6] to the PDI core. These reactions are being explored as a way to facilitate formyl formation and also to potentially use H2 as the reductant in the CO2-to-CO conversions on the FePDI scaffold.
Figure 2. ORTEP view of Fe(tBuBnzPDI)Br2. The ligand scaffold does not contain –CH3 or -CH(CH3)2 groups.
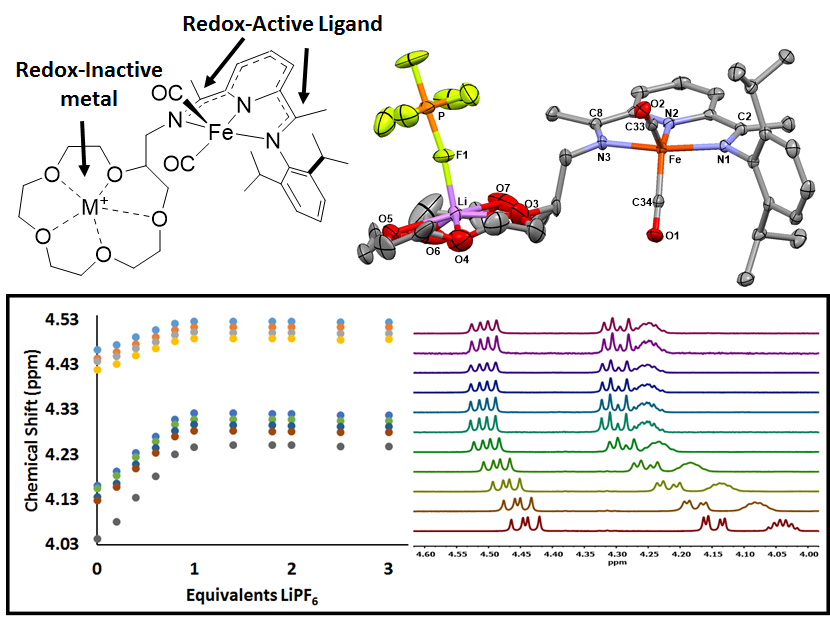
Lastly, in continuing with the theme of novel metal-ligand complexes based on the pyridinediimine (PDI) scaffold, we have successfully synthesized and characterized a series of complexes with pendant redox-inactive metals located in the secondary coordination sphere. Redox-inactive metals are often used in combination with redox-active transition metals in synthetic and biological systems to invoke reactivity involving electron transfer. The reduced dicarbonyl complex Fe(15c5PDI)(CO)2 was shown in both the solid state and solution to encapsulate redox-inactive metal ions, where the reduction potential of the metal-ligand scaffold was found to shift by ~50 mV upon encapsulation of either Na+ or Li+. That work has been submitted as a communication to Inorganic Chemistry and requested revisions are currently under review.
Figure 3. Graphic describing the structure of [Fe(15c5PDI)(CO)2Li][PF6] (top) and solution 1H NMR titration of Fe(15c5PDI)(CO)2 with LiPF6 in acetonitrile-d3.
Work on the last year of the grant will focus on the reactivity of all of the compounds and types of compounds described above.
There were two students that received summer stipends during the 14-15 reporting period (Kyle Burns and Pui Man Cheung). Kyle has graduated and is pursuing a Master’s degree in Chemistry at WWU. Pui Man Cheung (Audrey) will graduate in Fall 2015 and plans to pursue graduate school in Chemistry.