Reports: DNI1053668-DNI10: Synthesis of Nanostructured Multi-Metallic Carbide Materials for Inexpensive Catalysts and Catalyst Supports
Brian Leonard, PhD, University of Wyoming
In-depth study of monometallic carbides synthesis
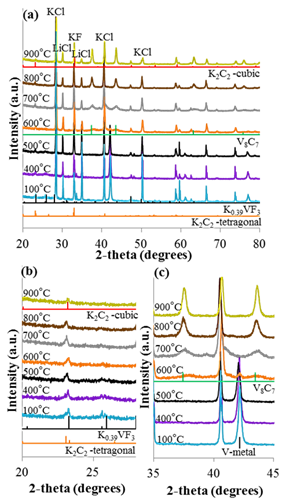
Difficulties forming bimetallic carbides using our salt flux method forced us to further study the reaction mechanism of this method to gain insight into the synthesis of bimetallic compounds. When trying to combine two metals, we found that the reactions temperatures, ratios of metals, type of salts, and annealing time all had somewhat unpredictable results. One of the most exciting results from this study was an ex-situ XRD method for monitoring the formation of carbide materials. By studying these reactions at 100°C intervals as seen in figure 1, we could determine the formation temperature of the carbide by watching the disappearance of the metal peak and the appearance of the carbide peaks. This is best seen in figure 1c at 600°C there is a dramatic loss of the V metal peak at ~42 and subtle peaks for V8C7 appearing at 37 and 43. Longer annealing at 600°C produced even more crystalline peaks of V8C7 thus helping establish the lowest temperature necessary for the formation of metal carbide materials. We followed this procedure for several other metals to determine ideal formation temperature ranges when combining multiple metals.
Figure 1. XRD Plots of (a) the reaction between vanadium metal and MWCNTs in salt flux at multiple temperatures. (b) highlights the formation and decomposition of K0.39VF3, K2C2-tetragonal, K2C2-cubic, and (c) shows the decomposition of V metal, and the formation of V8C7.
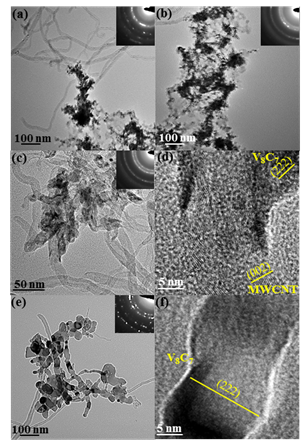
Figure 2. TEM images and SAED of the reaction between V metal and MWCNTs at (a & b) 500 ºC (c & d) 600 ºC and (e & f) 900 ºC
Additoinally, the TEM studies of these materials at 100°C intervals illustrates the formation of metal carbides from metal and MWCNTs into tube shaped carbides at 600°C and nanowires at 900°C. While completely understanding the reaction of the MWCNTs and metal is beyond the scope of this project, the understanding obtained in this brief study will provide insight into the methods to study carbide formation.
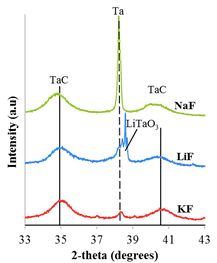
On final interesting finding of this study was the role of the salt cation in the formation process. As seen in figure 3, when KF is used as the reaction solvent, much more product forms than when using only NaF or LiF. These simple control reactions can be used when studying new phases/metal carbides to further understand how to optimize its reaction conditions for bimetallic carbide formation.
Figure 3. XRD plot of products from Ta and MWCNTs heated to 900 ºC in pure KF, LiF, and NaF.
Bimetallic carbides
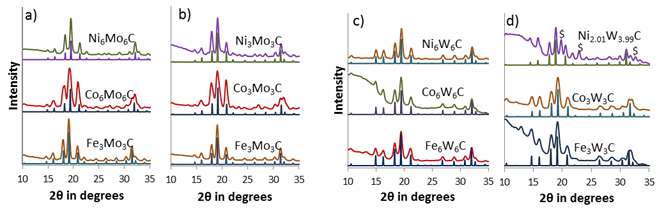
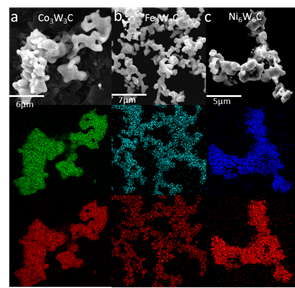
Another major result of this project was developing a reproducible way to create bimetallic carbides of the late transition metals (Fe, Co, Ni) with W and Mo. These carbides are highly sought after for catalysis studies but frequently suffer from phase separation and aggregation using traditional synthesis methods. As seen in figure 4, we were able to form bimetallic carbides of Fe,Co, and Ni with Mo and W. Further, we were able to control the crystal structure of these materials including control over the carbon content. We were able to consistently make phase pure samples with homogeneous composition throughout the nanomaterials as confirmed by EDX in figure 5.
Figure 4. XRD patterns of low (a) and high (b) carbon content bimetallic carbide phases of Ni, Co and Fe with Mo and low (c) and high (d) carbon content bimetallic carbide phases of Ni, Co and Fe with W.
Figure 5. EDX mapping high carbon content bimetallic carbide phases of Co3W3C (a), Fe3W3C (b) and Ni6W6C (c). Red map corresponds to tungsten while green, cyan and blue correspond to cobalt, iron and nickel respectively.
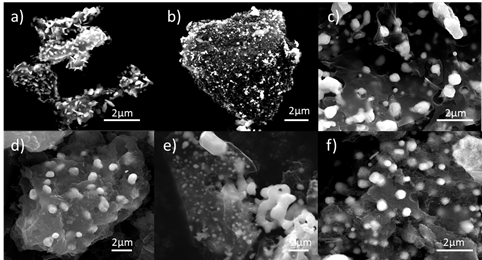
By controlling the amount of excess carbon used in the reactions, we can further control the size of the carbide products and whether or not they were supported on a carbon network. This level of particle size, composition, and crystal structure control has not been previously demonstrated for these bimetallic carbides and thus opens the door to further catalysis studies.
Figure 6. SEM images of bimetallic carbide particles of various systems dispersed in a network of carbon. a) Co3Mo3C b) Fe3Mo3C c) Ni3Mo3C d) Co3W3C e) Fe3W3C and f) Ni3W3C
Impact on PI and Students’ Careers
This PRF grant helped establish the PI’s research into metal carbide catalyst synthesis and now into the catalysis characterization. He has trained multiple students throughout the course of this grant and has published multiple papers. In addition, he and his students have given multiple presentations at ACS and Gordon Research conferences. The work done under this grant has produced the preliminary d
The primary student on this grant, Sami Schmuecker published her first paper ever which taught her many things about the discrimination of scientific results. She also presented her findings at two national ACS conferences (Dallas and Denver) with an poster and oral presentation respectively. She is currently finalizing her work at UW including 3 more publications, and will be graduating in 2016.
Cheng Wan and Yagya Regmi helped Sami with some of the synthesis of bimetallic carbides and learning different characterization techniques like Scanning Electron Microscopy (SEM). While Sami is quite independent, at times we needed results for publication or to continue progress in the project and this required addition help from other group members not funded by the project.
Ken Madsen is the latest addition to this work and started this spring working as an undergraduate. This is the first research project Ken has even undertaken and is learning all about scientific research including keeping a good lab notebook, new synthesis and characterization methods, and presentation skills during our weekly group meetings. He is a star undergraduate student and has already made great contributions to this project. He will be presenting his results at the spring 2016 ACS meeting.