Reports: UNI354419-UNI3: Mechanistic Investigations of Aryl-SO2--heteroatom Bond Formation from Palladium Using Bench-Stable SO2 Sources
Nicholas D. Ball, PhD, Pomona College
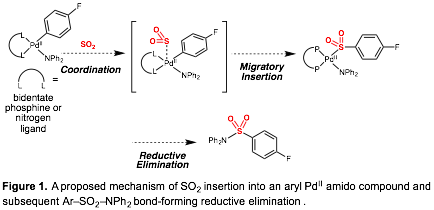
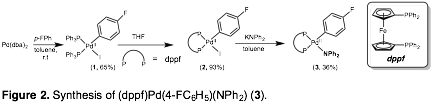
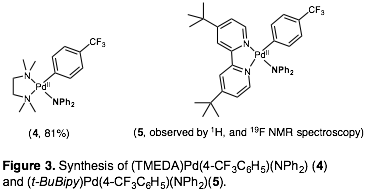
Nicholas D. Ball, PhD, Pomona College



Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society