Reports: UR354584-UR3: Antimony(V) Cations as Lewis Acid Catalysts for C-F Activation
Todd Hudnall, PhD, Texas State University
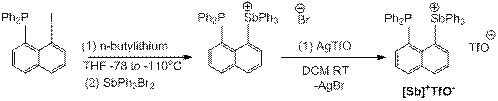
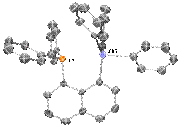
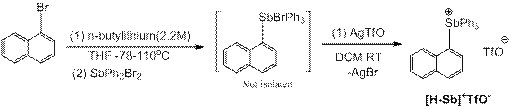
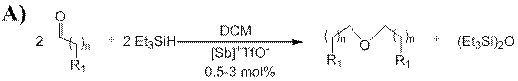

Todd Hudnall, PhD, Texas State University
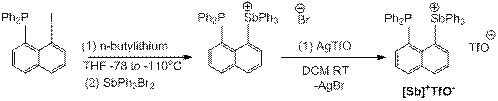


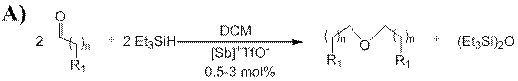

Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society