Reports: UNI952948-UNI9: Non-Catalytic Reactions Producing Phenols from Aromatic Hydrocarbons Using Hydrogen Peroxide and Supercritical and Subcritical Water
Jonathan E. Wenzel, PhD, Kettering University
Overview
Supercritical water is a highly energetic medium for partial and complete oxidation of hydrocarbons. Many non-polar compounds, such as benzene, are at least partially soluble in supercritical water owing to water’s low dielectric constant above water’s critical point of 374°C and 22.1 MPa. In addition, supercritical fluids have high diffusivities when compared to liquids and higher densities compared to gases, which can aid in over mass transfer hindrances. Supercritical water may be used to partially oxidize benzene with several studies evaluating the reaction kinetics and mechanisms of benzene oxidation that ultimately lead to gasification. This study focuses on optimizing the conversion of the supercritical water partial oxidation of benzene to phenol and other non-gaseous species non-catalytically.
During the second year of this project, a systematic study was initiated to determine the effects of varying temperature, reaction time, and initial benzene concentration upon conversion of benzene to partial oxidation intermediate products, such as phenol. The partial oxidation of benzene in 30 wt-% hydrogen peroxide was evaluated using an agitated 500 mL Hastelloy® C276 batch reactor between 390 and 430 °C with reaction times between 5 and 30 minutes, and initial benzene fractions of 1 and 2.5 mol-%. The principal products of the partial oxidation reaction at these conditions were, in order of decreasing abundance, phenol, biphenyl, benzoic acid, acetophenone, dibenzofuran, and trace amounts of xanthone. It was found that temperature and initial concentration of benzene was statistically significant upon yield of phenol when hydrogen peroxide density and water density was held constant. During the second year, experiments were performed to more closely evaluate the trends of conversion with temperature, reaction time, and initial benzene concentration. In addition, during the second year of this project, it was found that the method used to determine the methyl isobutyl ketone-water partition coefficients of the reactant and product species was inaccurate. MIBK is used to extract benzene and product species from the resultant ambient-condition hydrocarbon-water product mixture. The hydrocarbon content of the MIBK phase is quantitatively analyzed using a GC with FID. Determination of the MIBK-water partition coefficients will be completed during the no-cost extension period of this project. Reported mole-fractions in this report are without MIBK-water partition coefficients which will result in an underestimation of phenol and benzoic acid since these species will partition into water, unlike benzene and biphenyl.
Results
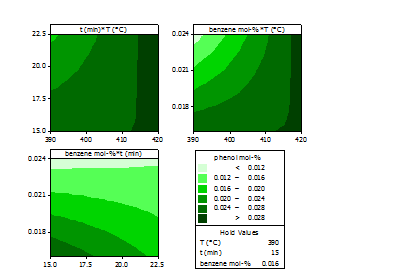
A 23 factorial experimental design was used as an aid to determine the significance of the main effects: temperature (390-420 °C), reaction time (15-23 minutes), and initial benzene mole fraction (1.5-3%). Temperature and initial mole fraction of benzene was statistically significant for phenol mole fraction with p≤0.05. Conversion to phenol is favored at lower mole fractions of benzene and higher temperature, Figure 1. The reported hydrocarbon mole fraction is the mole fraction of dissolved hydrocarbons extracted from the reaction products using MIBK, as well as unreacted benzene, all recovered at room temperature. It was found that biphenyl followed similar trends to that of phenol.
Figure 1: Contour plots of phenol mole fraction from a 23 factorial design with replicates, without MIBK-water partition coefficient.
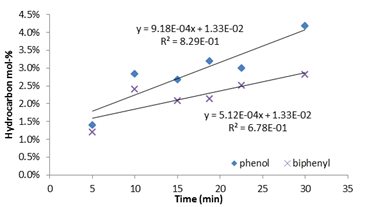
For year 2, the main effects of temperature, initial mol-% of benzene, and reaction time upon phenol and biphenyl product content were evaluated in more detail. It was found that as reaction time increased, the amount of phenol and biphenyl both increased, Figure 2. Since the slope for phenol is greater than that of biphenyl, it may indicate that the reaction producing phenol may be more time-dependent than that of biphenyl. This may be due to a variety of factors that are presently being studied and can potentially indicate the mechanism of the reactions that produce phenol using supercritical water with hydrogen peroxide as well as the reaction rate law.
Figure 2: Effect of reaction time upon phenol and biphenyl mol-%, without MIBK-water partition coefficient. Experimental Conditions: 7.5 mL (1.5 mol-%) benzene, 12.5 mL water, 87 mL 30 wt-% H2O2
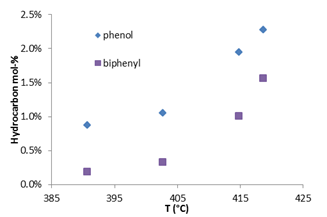
In addition, the amount of phenol and biphenyl produced using supercritical water and hydrogen peroxide was also evaluated as a function of temperature, Figure 3. As temperature increased from 390 to 420 °C, the amount of phenol and biphenyl correspondingly increased. It is important to note that as the reaction temperature increased, the final pressure of the reactor at room temperature did not significantly change. This indicates that the number of moles of gas produced during the course of the reaction did not change significantly over the range of temperatures studied.
Figure 3: Effect of temperature upon phenol and biphenyl mol-%, without MIBK-water partition coefficient. Experimental Conditions: Reaction time=15 min, 7.5 mL (1.5 mol-%) benzene, 12.5 mL water, 87 mL 30 wt-% H2O2
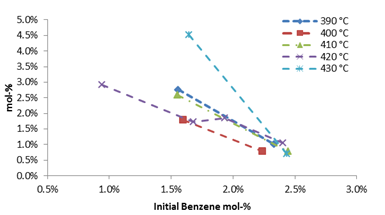
As the initial benzene mole fraction in the reactor was increased, the amount of phenol correspondingly decreased over a range of temperatures evaluated, Figure 4. Biphenyl exhibited a similar trend, however, the amount of benzoic acid increased with increasing amounts of benzene.
Figure 4: Effect of initial benzene mol-fraction upon phenol produced. Reaction time=15 min. Phenol hydrocarbon fraction without H2O/MIBK partition coefficient.
Student Impact
Exploratory experimental work was performed by undergraduate chemical engineering students for this project as part of a research elective class. This project has employed a chemical engineering and a biochemistry coop student for over four quarters. The measurement of MIBK-water partition coefficients is the subject of an undergraduate research thesis. Students have presented results of this work at Kettering University and Michigan Academy of Arts, Science, and Letters (MASAL) poster sessions.
Presentation of Results
Various results have been by undergraduate students at the 2013 and 2014 MASAL and at Kettering University’s homecoming celebration in the form of posters and oral presentations. Results were presented by the principal investigator at the 2014 American Institute of Chemical Engineers (AIChE) National Meeting.
Future Plans
The MIBK-water partition coefficients and a re-interpretation of the results of nearly 60 supercritical water partial oxidation experiments will be presented at the 2015 AIChE National Meeting. Future experiments will further evaluate reaction kinetics and results will be published.