Reports: UNI151029-UNI1: Calcium Catalyzed Homologous Conjugate Addition Reactions
Kristine Nolin, PhD, University of Richmond
Our group has been developing catalytic homologous conjugate addition (HCA) reactions, additions to electron-deficient cyclopropanes, utilizing calcium(II) complexes to facilitate the reactions. While many methods exist for catalytic 1,4-additions of nucleophiles to electron deficient conjugated ¹-systems, the homologous reaction has yet to be fully realized. HCA reactions are usually performed under forcing conditions using stoichiometric or super-stoichiometric amounts of a Brönsted base and/or Lewis acid. Unlike Lewis acid metals commonly used in synthetic methodologies, calcium complexes are relatively inexpensive and many are environmentally benign. The large radii and electropositive nature of calcium leads to a similarity in coordination behavior and observed reactivity to lanthanide metals.
Current Results
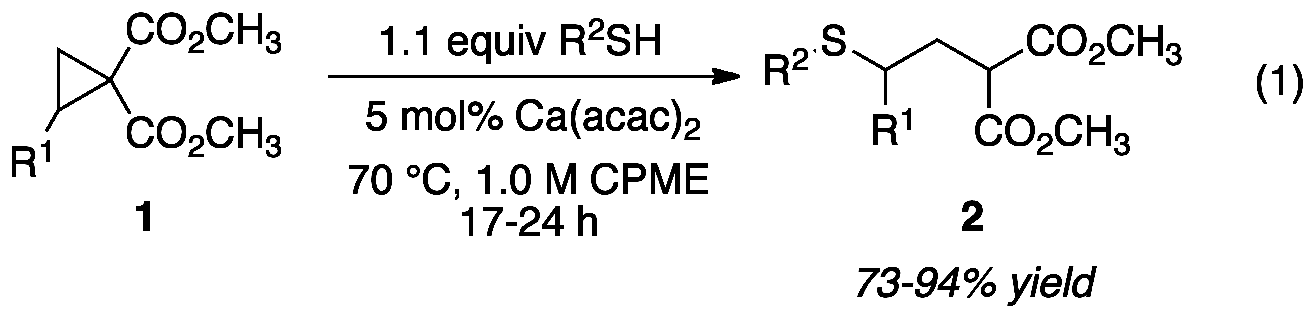 Development of methodology for the addition of unactivated thiols to donor-acceptor (DA) cyclopropanes has been completed. After a catalyst screen, we found that commerically available calcium acetylacetonate (Ca(acac)2) facilitated the addition of 4-chlorothiophenol, without prior activation or exogenous base, to cyclopropane 1 with conversion of 67% Reaction conditions were optimized and the reaction was probed for generality with regards to substitution on the thiols and the cyclopropane. Electron-rich and electron-deficient thiophenol derivatives as well as alkyl thiols added to provide the corresponding g-sulfanyl malonates in good to excellent yield (eq 1). The electronic structure of the DA cyclopropane had minimal impact on the productivity of the reaction. This work has been published.
Development of methodology for the addition of unactivated thiols to donor-acceptor (DA) cyclopropanes has been completed. After a catalyst screen, we found that commerically available calcium acetylacetonate (Ca(acac)2) facilitated the addition of 4-chlorothiophenol, without prior activation or exogenous base, to cyclopropane 1 with conversion of 67% Reaction conditions were optimized and the reaction was probed for generality with regards to substitution on the thiols and the cyclopropane. Electron-rich and electron-deficient thiophenol derivatives as well as alkyl thiols added to provide the corresponding g-sulfanyl malonates in good to excellent yield (eq 1). The electronic structure of the DA cyclopropane had minimal impact on the productivity of the reaction. This work has been published.
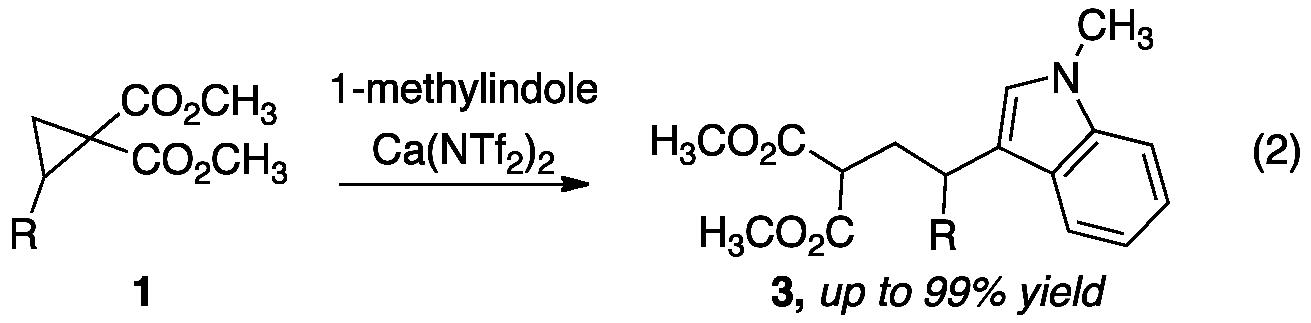 We have expanded upon our calcium-catalyzed addition reactions by developing catalytic C–C bond forming reactions. Two classes of carbon nucleophiles are of particular interest and are being tested as substrates in the HCA reaction: (1) electron-rich aromatic and (2) heteroaromatic compounds. We began our investigation into these catalytic Friedel-Crafts reactions by examining the addition of 1-methylindole to cyclopropane 1 (R=Ph) at room temperature in dichloromethane. A screen of commerically available calcium(II) complexes revealed that 10% calcium triflimide (Ca(NTf2)2) facilitated production of a modest amount of the corresponding Friedel-Crafts product 3 (R=Ph). By increasing the temperature to 70 °C and switching to 1,2-dichloroethane, the reaction proceeded with >99% conversion as determined by 1H NMR (eq 2). Chlorinated solvents can be avoided by using cyclopentyl methyl ether, CPME, in which the reaction proceeds with 86% conversion. Cyclopropanes bearing electron-rich and electron-deficient aryl substitutions underwent addition of 1-methylindole to provide the corresponding products in good to excellent yield (up to 99%). Heteroaromatic substituted indole products were also obtained in very good yield (87-93%). This work has been published.[2]
We have expanded upon our calcium-catalyzed addition reactions by developing catalytic C–C bond forming reactions. Two classes of carbon nucleophiles are of particular interest and are being tested as substrates in the HCA reaction: (1) electron-rich aromatic and (2) heteroaromatic compounds. We began our investigation into these catalytic Friedel-Crafts reactions by examining the addition of 1-methylindole to cyclopropane 1 (R=Ph) at room temperature in dichloromethane. A screen of commerically available calcium(II) complexes revealed that 10% calcium triflimide (Ca(NTf2)2) facilitated production of a modest amount of the corresponding Friedel-Crafts product 3 (R=Ph). By increasing the temperature to 70 °C and switching to 1,2-dichloroethane, the reaction proceeded with >99% conversion as determined by 1H NMR (eq 2). Chlorinated solvents can be avoided by using cyclopentyl methyl ether, CPME, in which the reaction proceeds with 86% conversion. Cyclopropanes bearing electron-rich and electron-deficient aryl substitutions underwent addition of 1-methylindole to provide the corresponding products in good to excellent yield (up to 99%). Heteroaromatic substituted indole products were also obtained in very good yield (87-93%). This work has been published.[2]
A direct extension of this methodology is underway examining the addition benzofuran to DA cyclopropanes. We have not found precedence for the reaction in the literature and have been excited by our initial results. The benzofuran reaction has different regioselectivity than the indole addition described above. In addition, there is a highly competitive cycloaddition reaction. We have yet to determine optimal reaction conditions that would be selective for the Friedel-Crafts or cycloaddition pathway. We have a collaboration with Dr. Carol Parish and an undergraduate in her lab is performing quantum mechanical studies of these reactions.
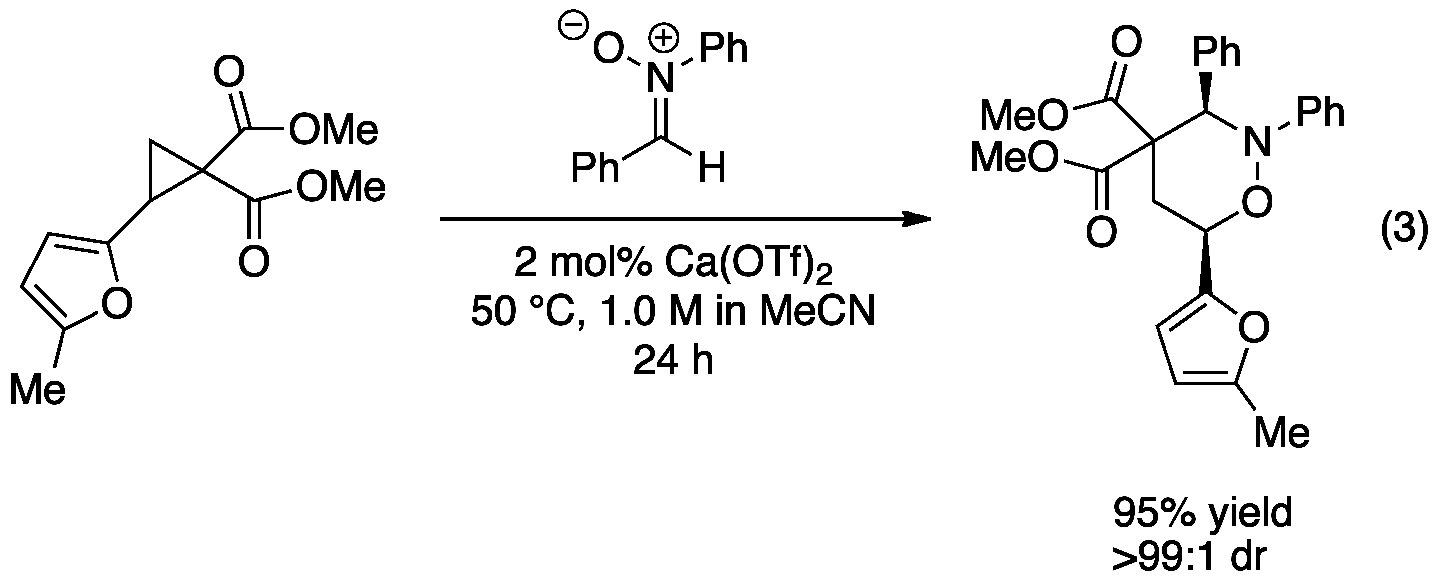 Our activation of cyclopropanes using calcium(II) complexes has expanded into cycloaddition reactions. We recently published our new method for catalyzing the 1,3-dipolar cycloaddition of nitrones and DA cyclopropanes using commercial available calcium triflate (eq 3).[3] The reaction shows generality of substitution on the cyclopropane as well as on the nitrone. A range of tetrahydro-1,2-oxazines were isolated in good to excellent yield with high levels of diastereoselectivity in favor of the cis diastereomer. This methodology is currently being expanded to include the 1,3-dipolar cycloaddition of nitrones and polarized alkenes.
Our activation of cyclopropanes using calcium(II) complexes has expanded into cycloaddition reactions. We recently published our new method for catalyzing the 1,3-dipolar cycloaddition of nitrones and DA cyclopropanes using commercial available calcium triflate (eq 3).[3] The reaction shows generality of substitution on the cyclopropane as well as on the nitrone. A range of tetrahydro-1,2-oxazines were isolated in good to excellent yield with high levels of diastereoselectivity in favor of the cis diastereomer. This methodology is currently being expanded to include the 1,3-dipolar cycloaddition of nitrones and polarized alkenes.
Impact
ACS-PRF support has enabled four research students to have a summer research experience and a dozen more to have supplies for research during the school year. Some of these students have graduated and are continuing their studies toward PhD, MD, and DDS degress. These students have made tremendous progress on their projects and were able to gain experience in reaction optimization, synthesis, purification methods, instrumentation usage, and data analysis. Their results were communicated in publications and numerous presentations including an award-winning poster presentation at 2012 SERM-ACS meeting.
[1] Braun, C. M.; Shema, A. M.; Dulin, C. C.; Nolin, K. A., "Homologous Conjugate Addition of Thiols to Electron Deficient Cyclopropanes Catalyzed By a Calcium(II) Complex" Tetrahedron Lett. 2013, 54, 5889–5891.
http://dx.doi.org/10.1016/j.tetlet.2013.08.102
[2]Dulin, C. C.; Murphy, K. L.; Nolin, K. A., ÒCalcium-Catalyzed Friedel-Crafts Addition of 1-Methylindole to Activated CyclopropanesÓ Tetrahedron Lett. 2014, 55, 5280–5282. http://dx.doi.org/10.1016/j.tetlet.2014.07.108
[3] Braun, C. M.; Congdon, E. A.; Nolin, K. A. Diastereoselective 1,3-Dipolar Cycloaddition of Nitrones to Donor-Acceptor Cyclopropanes Catalyzed by a Calcium(II) Complex. J. Org. Chem. 2015, 80, 1979–1984. pubs.acs.org/doi/abs/10.1021/jo502686t











