Reports: UR551765-UR5: Atomistic Simulations of Small Molecules' Behavior Within Al Substituted Zeolites
Daniela Kohen, Carleton College
We continue to make progress in understanding and characterizing at the molecular level how small gas molecules with and without a strong quadrupole behave within pores of molecular sieves. Micro-porous and nano-porous materials are widely used in catalytic and separation processes. Our atomistic simulations provide basic scientific insights that complement experiments and facilitate the development of new materials that can both be effective and inexpensive. In particular, we are now studying gases’ behavior within Al-substituted zeolites. When Al atoms are introduced into an all-silica zeolite the framework becomes negatively charged which necessitates the presence of mobile cations within the cavities of the zeolites. These cations greatly impact the ability of the material to absorb gases. Atomistic simulations can aid by accurately predicting how cations’ behavior changes in the presence of different absorbed gases. This is especially important in materials where preferred sites are within narrow necks connecting cages.
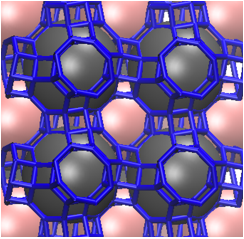
For example, some zeolites are known to experience a phenomenon called “cation gating” which allows carbon dioxide but not other sorbents to permeate the zeolite, giving rise to very high adsorption selectivities for CO2. This last year we studied the behavior of CO2 within one such zeolite, Na-Rho (figure 1).
Figure 1. The structure of zeolite Na-RHO when P CO2= 1 bar.[20] The framework is shown in blue. Note the 3-dimensional channel system composed of cavities (-cages). Each -cage is connected to six others by double eight rings (D8Rs). This gives rise to two interpenetrated but not interconnected pore systems (shown in grey and pink).
We have been able to provide further insight into the reasons behind this intriguing phenomenon. We showed that CO2's favorable electrostatic interactions with the zeolite framework result in preferential binding in the opening of the channels between cages. This lead us to suggest a novel mechanism to explain carbon dioxide's unique “gate opening behavior” in which this preference for binding inside the “gate” allows CO2 to “squeeze” by the gate-keeping cation as it moves around slightly due to thermal fluctuations. This proposed mechanism is distinct from a previously proposed mechanism in which carbon dioxide mediates the displacement of gate-keeping cations via electrostatic interactions, and may be in better agreement with experimental evidence.
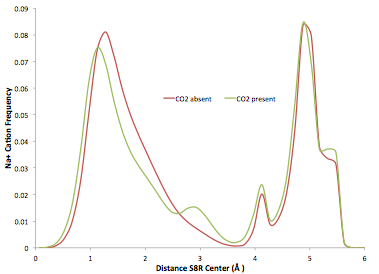
To reach these conclusions, we first investigated the equilibrium positions of cations in the presence and absence of carbon dioxide using radial density probability functions. Figure 2 shows that the distributions do not vary much between when carbon dioxide is present and when it is not. However, there is a net movement of cations away from the center of the 8-rings when CO2 is introduced into the zeolite, a finding that is in agreement with the experimental observation that CO2 is not blocked by cations.
Figure 2. Cation radial probability density functions. Note that the distributions do not differ substantially between when carbon dioxide is present and when it is not. In both cases there is a peak near zero corresponding to a single eight ring (S8R) site and another much further away corresponding to cations on the S6R site. However, while the distribution of cations in the S6R is essentially unchanged, there is a small shift in the distribution of cations in the S8R when carbon dioxide is present: the probability of a cation being between 1-2 from the center of the ring is smaller while around 3 the probability is larger than in the absence of the adsorbate.
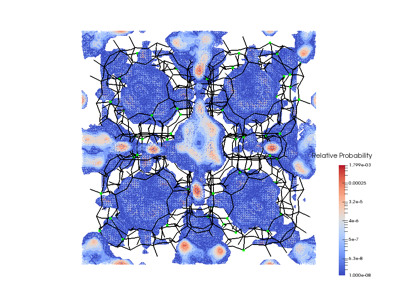
To address the issue of what causes the cations to move from their blocking positions and allow for the “opening of the gate” we computed probability maps for cations and carbon dioxide. These very clearly show that both cations and carbon dioxide have a preferred site of adsorption near the center of the S8R. They suggest an alternative explanation for carbon dioxide's ability to permeate the zeolite: perhaps the gating cation periodically moves around of the S8R by itself due to thermal motion, and CO2 is simply more inclined than other molecules to “squeeze” by into the S8R when this happens due to its natural affinity for this site. In other words, it is not so much that the carbon dioxide molecule opens the gate by interacting with the “gate keeper” sodium, but rather that carbon dioxide is able to take advantage of a “wandering gate keeper.” Within this alternative explanation, a methane molecule, for example, would not be able to take advantage of the cation's thermal motion because in the absence of strong electrostatic interactions with the pore it might lack carbon dioxide's preference for an S8R site.
Figure 3. Probability map for carbon dioxide. The figure shows a 2x2x2 simulation cell. The framework is shown in black with the Al atoms in green. The figure highlights the cages and the narrow passages in between then.
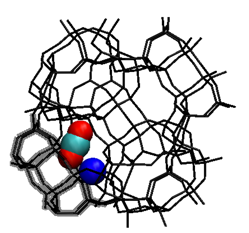
Our proposed mechanism is strengthen by our finding of an instance of a carbon dioxide entering a cation-blocked narrow channel between two a-cages (see figure 4). This strongly suggest that gases that do not possess a quadrupole cannot diffuse through S8Rs because they have no energetic reason for entering the narrow channel when then the cation is not quite blocking the S8R.
Figure 4. A snapshop of a carbon dioxide molecule entering a D8R blocked by a cation. Only the relevant adsorbate molecule and cation are shown in the figure. The blocked D8R is highlighted.
This last year our work has resulted in a poster at the Porous Materials Workshop (CPM-7), a talk at the regional MU3C meeting and an invited talk at the national Foundations of Molecular Modeling and Simulations Conference (FOMMS) meeting. We have also submitted an article describing this work to the FOMMS proceedings that will be published in the new book series "Molecular Modeling and Simulation: Applications and Perspectives."