Reports: ND153146-ND1: New Approaches to Alkene Functionalization via Dehydrogenative Metallation Catalyzed by First-Row Transition Metals
Jennifer M. Schomaker, PhD, University of Wisconsin (Madison)
Introduction. Our group has been engaged in uncovering new methods to promote the efficient and flexible functionalization of alkenes. While many such methods are known, the predominant focus to date has been on the use of precious metal catalysts (Pd, Pt, Rh, Ir). Recently, there has been increased emphasis on employing earth-abundant and more environmentally friendly first-row transition metal catalysts to carry out important transformations currently promoted by rare-earth metals. One strategy to accomplish this goal is to mimic the same mechanistic patterns that have been observed for second- and third-row metals; however, we have chosen to take a different approach. Our work takes advantage of the distinctive electronic structures of first-row metals to reveal completely new reactivities. In this project, we have designed a series of new copper catalysts capable of transforming simple alkene precursors derived from petroleum feedstocks into useful synthetic building blocks through an unusual 1,3-halogen migration/functionalization cascade.
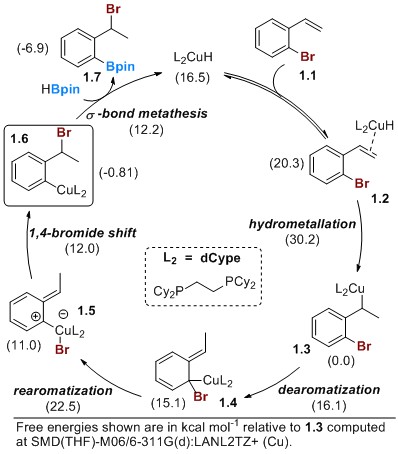
Ascertaining the mechanism through computational studies. Since the last reporting period, we have refined our initial computational studies to better understand the mechanism of the Cu(I)-catalyzed 1,3-halogen migration reaction (Scheme 1). All structures throughout these studies were optimized with Gaussian 09 using the M06 functional with a 6-311G* basis set for H, B, C, O, P, and Br and a LANL2TZ+ basis set for copper. An SMD continuum model was used with THF as the solvent. All minima were checked for absence of imaginary vibrational modes and all transition states were checked for one imaginary vibrational mode and confirmed with intrinsic reaction coordinate (IRC) calculations. These computations show that coordination of a copper hydride to the styrene is followed by regioselective hydrometallation to yield a benzyl copper species. The unexpected dearomatization/rearomatization sequence is followed by a 1,4-bromide shift to yield the key aryl Cu(I) intermediate. A final σ-bond metathesis with HBpin yields the observed product and generates the active copper hydride. The key to preventing competing alkene hydroboration is to ensure that the dearomatization pathway of 1.3 to 1.4 is lower in energy than direct borylation of 1.3.
Scheme 1. Proposed mechanism of 1,3-halogen migration/borylation elucidated through computational studies.
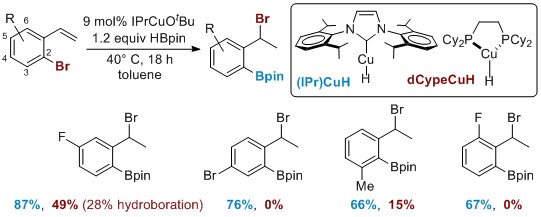
Improving the scope of the Cu-catalyzed 1,3-halogen migration. Several limitations in the substrate scope of 1,3-halogen migration promoted by copper catalysts supported by phosphine-based ligands prompted us to develop more robust catalysts for 1,3-halogen migration/functionalization. The major problems with employing 1,2-bis(dicyclohexyl-phosphino)ethane (dCype) as the ligand include competing hydroboration with e-poor systems, poor reactivity with ortho-substituted arene substrates and the lack of reactivity with aryl chlorides and heterocycles. In our investigations to address these issues, we found that NHCs were excellent ligands for Cu-catalyzed 1,3-halogen migration; indeed, a comparison of (IPr)CuH to dCype (Scheme 2) showed no competing hydroboration and significantly increased yields of the halogen migration for both e-poor arenes and those containing a variety of ortho substituents.
Scheme 2. New NHC-based catalysts for 1,3-halogen migration.
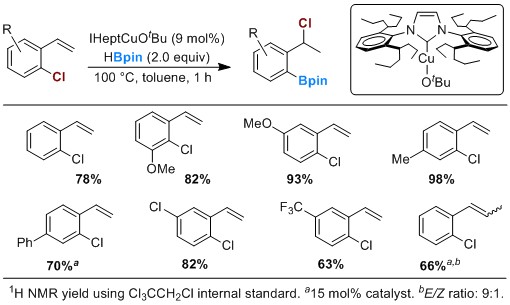
The results noted in Scheme 2 encouraged us to further pursue the development of thermally stable NHC-supported Cu catalysts to promote challenging 1,3-chloride migrations. This was an important goal for expanding the utility of our chemistry due to the ready availability and low cost of aryl chlorides, as well as the increased stability of the products of 1,3-chloride migration. We found that replacing the isopropyl group of IPr with the bulkier group of (IHept)CuOtBu (Scheme 3) permits reaction temperatures of up to 100 °C and enables ready transfer of Cl from an aryl carbon to a benzylic carbon.
Scheme 3. A thermally robust catalyst, IHeptCuOtBu, for 1,3-chloride migration.
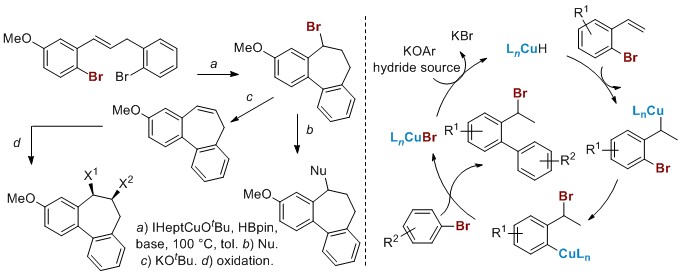
Tandem Cu-catalyzed 1,3-halogen migration/Suzuki coupling reactions. The development of thermally robust NHC-supported Cu catalysts has enabled us to achieve tandem reactions at elevated temperatures that degraded our first-generation catalysts. Key to these new methodologies are our computational and experimental studies showing that an aryl Cu(I) species is a key intermediate produced as a result of 1,3-halogen migration. The limited reactivity reported for such species presents an exciting opportunity to develop new applications of our copper chemistry. In this vein, we have recently observed an intramolecular tandem 1,3-halogen migration/Suzuki coupling (Scheme 4) that yields fused ring scaffolds that contain either a halogen or alkene functionality positioned for further elaboration. Current investigations are focused on expanding the scope of this reaction, rendering the halogen migration enantioselective and developing intermolecular versions of this reaction that are tantamount to formal halide-halide couplings.
Scheme 4. Tandem Cu-catalyzed 1,3-halogen migration/Suzuki coupling.
Impact of funding on students involved in this research. The students supported by this research grant have received a very broad training experience, gaining skills in a number of crucial areas, including organic synthesis, methodology development, catalyst design, the determination of reaction mechanisms, the development of predictive models for catalyst design and computational chemistry. Graduate students have assisted in manuscript preparation and participated in writing two perspective articles describing advances in 1,3-halogen migration and dyotropic rearrangements in general. Students involved in this project have presented their work at several national ACS meetings, several departmental seminars and the 2015 AbbVie symposium. Undergraduates involved in the project have presented at ACS meetings and departmental poster sessions and have gone on to attend top Ph.D. and D.D.S. programs at UC-Irvine and the University of Michigan. The preliminary results that were obtained through the generous support provided by the ACS-PRF ND program were included in a full proposal on this work that was submitted to the NIH this past June.