Reports: ND1052674-ND10: Volatile Heterometallic Molecular Precursors for Oxide, Fluoride, and Silicate Cathode Materials of Thin Film Lithium Ion Batteries
Evgeny V. Dikarev, State University of New York at Albany
Overview
This project is focused on the development of heterometallic molecular compounds that contain lithium (sodium) and transition metal atoms and can be utilized as volatile single-source precursors in preparation of thin films and nanocrystals of rechargeable battery cathode materials. Our group is designing heterometallic precursors that are stable, resistant to hydrolysis and exhibit clean, low-temperature decomposition pattern as well as the proper metal:metal ratio for the target material.
Heterometallic Molecular Precursor for the Preparation of Low-Temperature Lithium-Manganese Oxide Phase, a Prospective Cathode Material
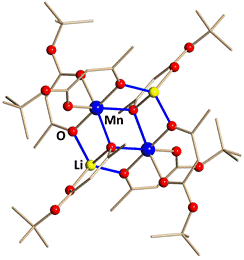
We have already established that heterometallic compounds with tert-butyl acetoacetate (tbaoac) ligand are capable to yield phase-pure mixed-metal oxides upon thermal decomposition at relatively low temperatures. In this work, we successfully used the ligand to obtain heterometallic molecular precursor with the Li:Mn = 1:1 metal ratio. Compound can be isolated with nearly quantitative yield on a large scale. Single crystal X-ray investigation confirms the presence of tetranuclear molecules Li2Mn2(tbaoac)6 (Figure 1).
Figure 1. Solid state structure of heterometallic precursor Li2Mn2(tbaoac)6. The metal–oxygen bonds involved in bridging interactions are shown in blue. Hydrogen atoms are omitted for clarity.
Asymmetric tert-butyl acetoacetate features small/bulky substituents at the opposite ends of the ligand that results in different bridging connectivity properties at the two coordinating oxygen sites and allows one to design the heterometallic assembly with discrete molecular structure. Only one of the ligand oxygens, located under the methyl substituent, is participating in bridging of metal atoms, while the other one, placed under the sterically congested tert-butoxy group, is simply chelating. These bulky ends of the ligand are facing outward of heterometallic assembly thus providing an additional screening protection as well as preventing intermolecular bridging contacts and the formation of a polymeric structure.
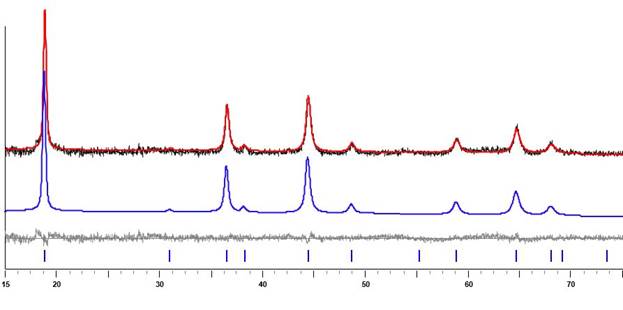
In accord with its structure, Li2Mn2(tbaoac)6 is highly volatile and soluble in nearly all common solvents, while retaining its heterometallic core in solution. Thermal decomposition of heterometallic precursor in air at the temperatures of ca. 400 oC results in a phase-pure lithium manganese oxide, Li1.5Mn1.5O3.5 (Figure 2). Structural investigation by high-resolution electron microscopy, X-ray powder diffraction, and XPS revealed that the product has an oxygen-deficient spinel structure. Electrochemical testing of the oxide material shows a good reversibility in the voltage range of 2.5-3.85 V (vs. Li+/Li).
Figure 2. X-ray powder diffraction pattern of Li1.5Mn1.5O3.5 obtained by decomposition of Li2Mn2(tbaoac)6 precursor in air at 400 oC and the Le Bail fit for the cubit unit cell (sp. gr. Fd-3m, a = 8.1400 Å.)
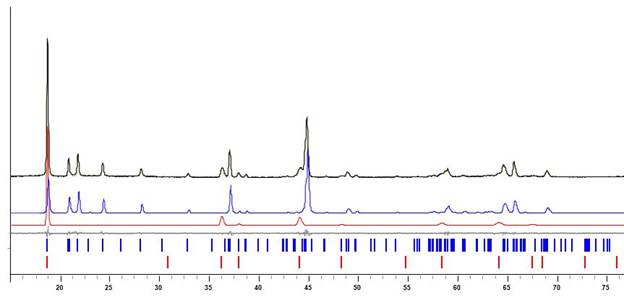
Increase of thermolysis temperature or annealing time lead to decomposition of the low-temperature phase and appearance of different oxide products: spinel LiMn2O4 and monoclinic Li2MnO3 (Figure. 3). Application of the single-source precursor Li2Mn2(tbaoac)6 having low-temperature decomposition proved to be crucial for the preparation of the above Li:Mn = 1:1 oxide phase. A number of analogous heterometallic precursors having higher decomposition temperatures were shown to afford the mixtures of lithium-manganese oxides.
Figure. 3 X-ray powder diffraction pattern (black) of the residues obtained by decomposition of Li2Mn2(tbaoac)6 precursor in air at 750 oC. Blue and red are calculated diffraction patterns for Li2MnO3 and LiMn2O4, respectively. The theoretical peak positions are shown at the bottom.
Design of Volatile Heterometallic Precursor for the Sodium Manganate Cathode Material
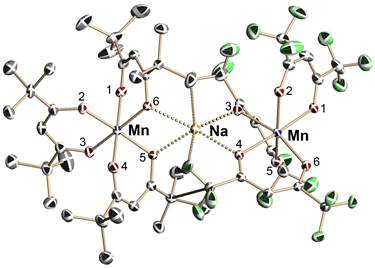
In this work, we were focused on design of heterometallic sodium-manganese single-source precursor with 1:2 ratio for prospective sodium ion battery cathode material Na4Mn9O18 (Na0.44MnO2). Attempts to synthesize the complex analogous to the previously reported Li-Mn heterometallic precursor LiMn2(thd)5 were unsuccessful, since sodium requires coordination number of more than four. In order to solve this problem, we utilized a mixed-valent approach to obtain heterometallic molecular precursor with the proper ratio for the target manganate material. Mixed-valent MnII/MnIII heteroleptic complex [Mn(thd)3]Na[Mn(hfac)3] was prepared with a high yield using one-step reaction procedure. In the trinuclear structure of precursor (Figure 4), the “naked” sodium ion is coordinated by two oxygen atoms of [Mn(thd)3] fragment as well as by two oxygen and fluorine atoms of [Mn(hfac)3] unit.
Figure 4. Molecular structure of mixed-valent heterometallic precursor [MnIII(thd)3]Na[MnII(hfac)3]. The Na–O bonds and Na–F interactions are shown as dashed lines. Hydrogen atoms are omitted for clarity.
The results of X-ray structural investigation allow one to make an unambiguous assignment of Mn atom oxidation states. The average Mn–O bond distance in the [Mn(hfac)3] fragment (2.157 Å) is considerably longer than that in the [Mn(thd)3] unit (1.991 Å). In addition, two axial Mn–O bonds in the [Mn(thd)3] octahedron are substantially (ca. 0.12 Å) longer than four equatorial ones, thus reflecting Jahn-Teller distortion typical for d4 Mn ion. Therefore, the complex was formulated as {[MnIII(thd)3]Na+[MnII(hfac)3]-}. Electron-rich Mn(II) center is chelated by diketonate ligand with electron-withdrawing CF3 groups, while Mn(III) ion bears ligands with electron-donating tBu substituents. Such a ligand distribution proved to be crucial for the formation of heterometallic complex.
Table 1. Mn–O Bond Distances (Å) in the Structure of Heterometallic Precursor [MnII(hfac)3]Na[MnIII(thd)3]
|
[MnIII(thd)3] |
|
[MnII(hfac)3]– | ||
|
Mn–O1 |
1.9228(2) |
|
Mn–O1 |
2.1449(3) |
|
Mn–O2 |
2.1480(3) |
|
Mn–O2 |
2.1374(2) |
|
Mn–O3 |
1.9138(2 |
|
Mn–O3 |
2.1405(2) |
|
Mn–O4 |
1.8829(2) |
|
Mn–O4 |
2.1683(3) |
|
Mn–O5 |
2.1330(3) |
|
Mn–O5 |
2.1781(2) |
|
Mn–O6 |
1.9481(2) |
|
Mn–O6 |
2.1751(3) |
|
(Mn–O)av |
1.991 |
< |
(Mn–O)av |
2.157 |
In accord with its molecular structure, the complex was found to be soluble in nearly all common solvents. It is highly volatile and exhibits a congruent sublimation with heterometallic fragments present in the gas phase. Heterometallic precursor demonstrates significantly lower moisture sensitivity than its homometallic components, which allows one to conveniently handle it in the course of decomposition studies.