Reports: ND353977-ND3: Photo-Functional Switches and Metal Organic Frameworks Containing Azobenzene Groups
Shawn Burdette, PhD, Worcester Polytechnic Institute
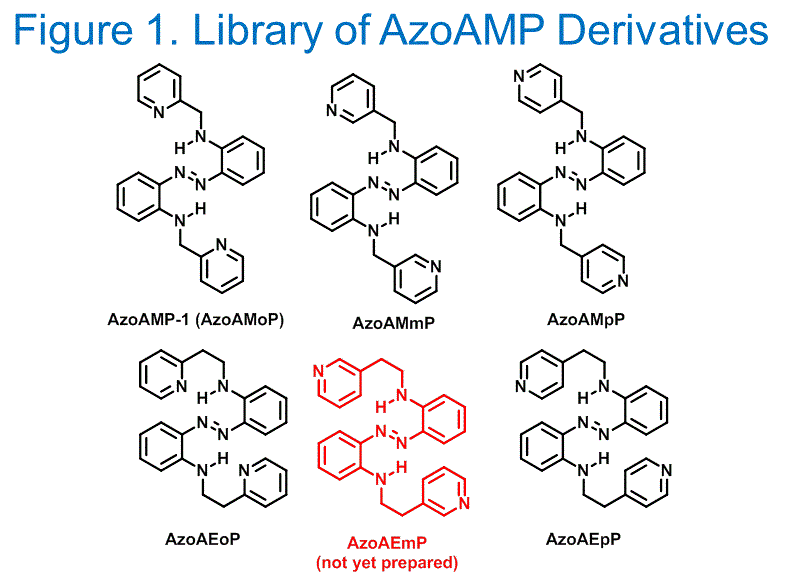
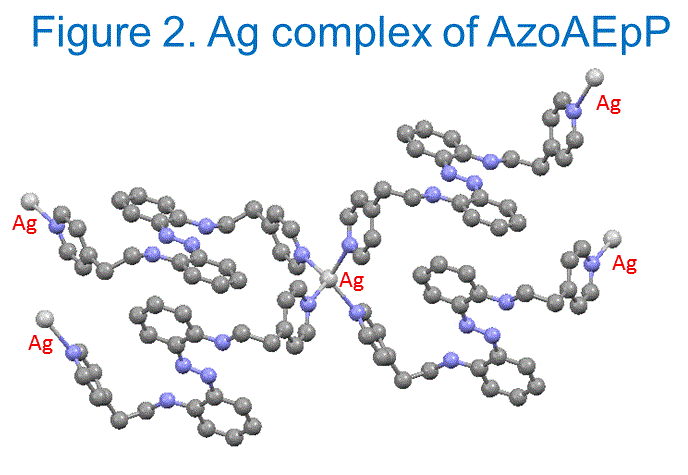
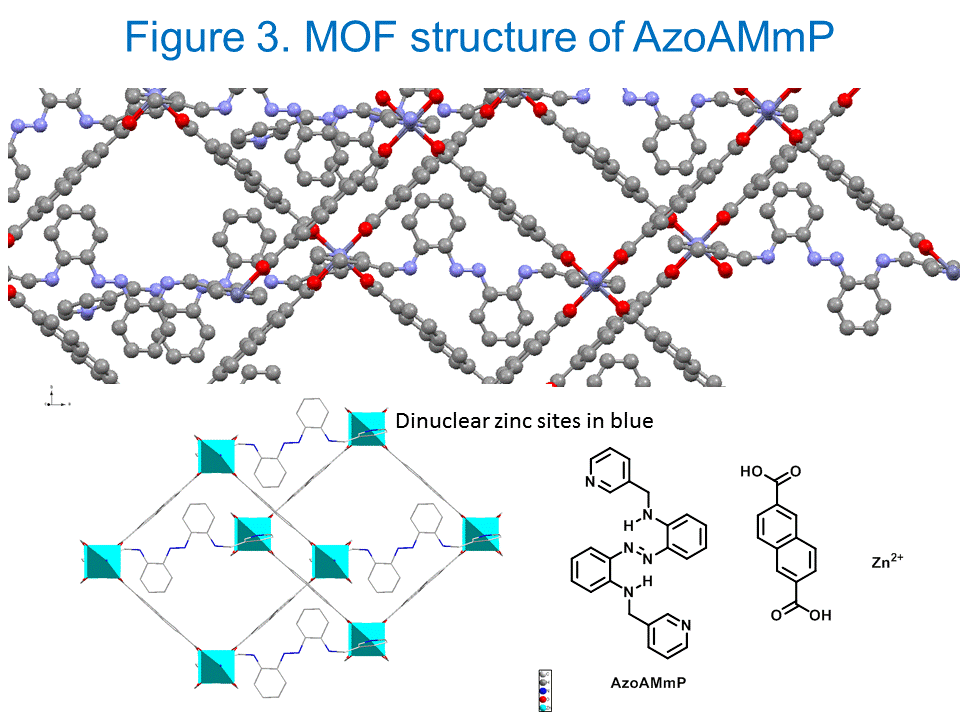
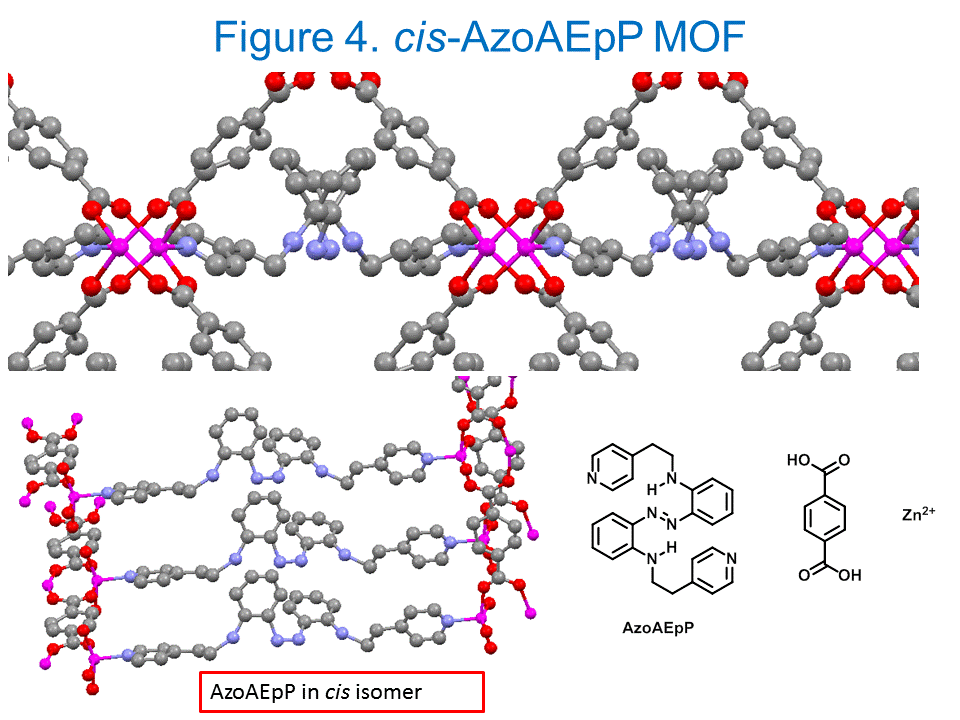
Shawn Burdette, PhD, Worcester Polytechnic Institute




Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society