Reports: ND653676-ND6: Ultrafast Photochemical Reactions in Cyclic Monoalkenes
Andrew M. Moran, University of North Carolina
I. Overview
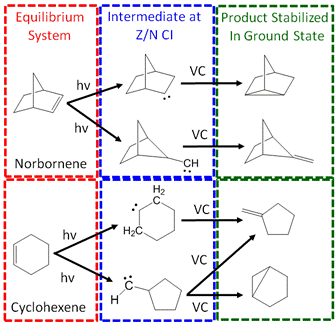
The goal of this project is to use newly developed sources of femtosecond deep ultraviolet laser pulses to study mechanisms of elementary chemical reactions. In recent years, our group has developed methods for generating sub-100-fs ultraviolet laser pulses at 200 nm using nonlinear processes in noble gases. Light at 200 nm is required to initiate ring opening reactions in cyclic monoalkenes such as cyclohexene and norbornene (see Figure 1). Studies of such elementary reactions may yield insights into the mechanisms at work in combustion processes.
II. Progress to Date IIA. Ultrafast Dynamics in Norbornene and Cylcohexene The experiments conducted in this project aim to understand
the ring opening mechanisms in cylcohexene and norbornene (see Figure 1). To
this end, femtosecond pump-probe experiments have been conducted with 200-nm
laser pulses. Femtosecond laser spectroscopies are challenged by undesired
photoionization processes and dispersion management in this wavelength range. Photoionization
processes must be suppressed by keeping the laser fluence very low, which makes
the signals small. In addition, the polarizability response of the solvent
dominates the signal when the pump and probe pulse are overlapped, thereby
obscuring information about internal conversion at pulse width limited delay
times (less than 200 fs in our setup). For this reason, extremely short lasers
pulses must be employed to resolve the internal conversion dynamics in these
systems.
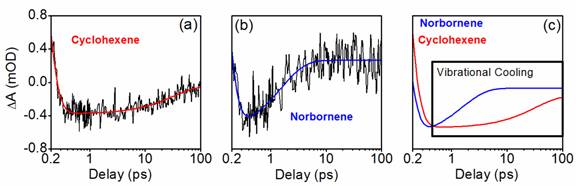
We have succeeded in detecting the vibrational cooling
dynamics that take place subsequent to internal conversion in both cyclohexene
and norbornene. The transient absorption data shown in Figure 2 are consistent
with a significantly faster vibrational cooling/product stabilization process
in norbornene. The mechanism responsible for this difference in behaviors is
unclear at this point. Dynamics at delay times less than 200 fs are hidden by
the response of the cyclohexane solvent. It appears that the internal
conversion process may be slightly faster in norbornene; however, we intend to
repeat these experiment with better time resolution in the fall of 2015.
Figure 2. Transient absorption data
obtained with 200-nm pump and probe pulses for (a) cyclohexene and (b)
norbornene in cyclohexane solvent. (c) The comparison of fits obtained for the
two systems suggests that internal conversion-induced vibrational cooling is
faster in norbornene than it is in cyclohexene. It appears that the internal
conversion processes take place within the first 0.5 ps following excitation.
Future work will attempt to improve the time resolution of this apparatus and
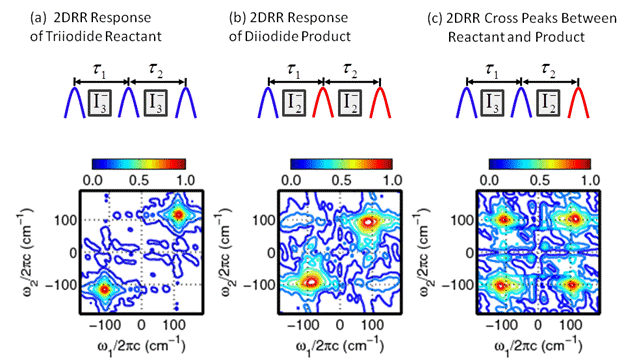
obtain better information about the internal conversion mechanisms. IIB. Photodissociation of Triiodide The setup we developed for generating the 200-nm laser
pulses also yields high-energy 267-nm laser pulses. These 267-nm pulses were
used to study the photodissociation reaction of triiodide with a new
experimental method, two-dimensional resonance Raman (2DRR) spectroscopy. 2DRR
spectroscopy yields insights into chemical reactions that cannot obtained using
conventional time-resolved approahes. With acknowledgment of ACS PRF support, two
applications of 2DRR spectroscopy were published in the Journal of Chemical
Physics in 2014-2015. One of these articles was received the honor of an
Editor's choice for 2014 (Molesky et al. J. Chem. Phys. 141, 114202, (2014)).
III. Impact of ACS-PRF Support on Personnel
IV. Future Directions