Reports: ND554149-ND5: Impact of Microenvironment on the Catalytic Activity of Supported Gold Nanoclusters
Jennifer Shumaker-Parry, PhD, University of Utah
Ilya Zharov, PhD, University of Utah
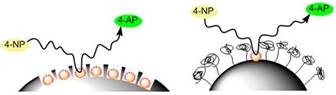
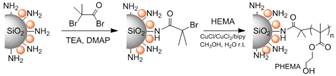
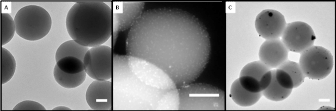
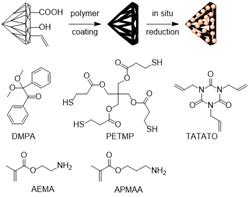
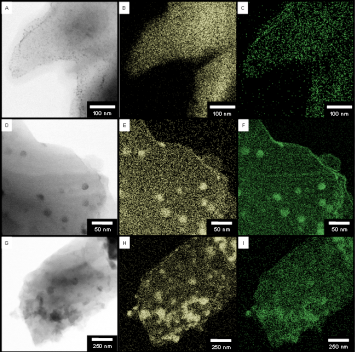
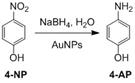
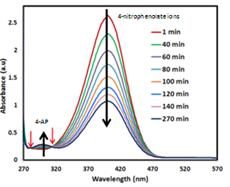
Jennifer Shumaker-Parry, PhD, University of Utah
Ilya Zharov, PhD, University of Utah







Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society