Reports: ND354739-ND3: New Ligands for Catalysis Through Elaboration of the Phosphatriptycene Framework
Joseph P. Sadighi, PhD, Georgia Institute of Technology
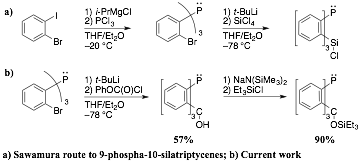
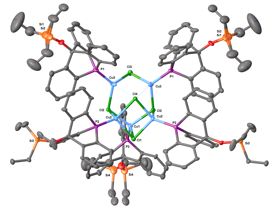
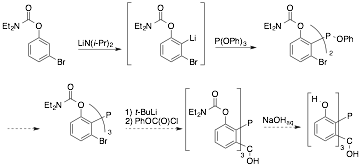
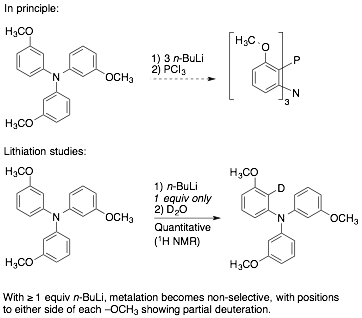
Joseph P. Sadighi, PhD, Georgia Institute of Technology




Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society