Reports: ND554543-ND5: Metal-Organic Frameworks as Gas-Phase Heterogeneous Catalysts: Understanding Active Sites for Oxidation and Hydrogenation Reactions
Donna A. Chen, University of South Carolina
Natalia B. Shustova, PhD, University of South Carolina
Overview
We are investigating metal-organic frameworks (MOFs) for the development of new catalytic materials, given that MOFs provide the ability to precisely control the architecture of reactive metal sites. The reactive metal centers in MOFs consist of unsaturated metal sites (UMS), and the exploration of chemical activity at these UMS is critical for understanding catalysis on MOFs. In the first stage of research for this grant, we have developed a systematic and integrated approach that combines optimization of MOF ligand/node design and synthetic methodology (PI: Shustova) with X-ray photoelectron spectroscopy (XPS) studies for characterization of MOF UMS (PI: Chen). Our initial studies have focused on two Cu-containing MOFs, and we have achieved the following the following key results: extensive reduction of Cu+2 to Cu+1 at the MOF metal nodes while retaining the original MOF structure, and selective binding of adsorbates as a function of the UMS oxidation state. A manuscript was submitted to the Journal of Physical Chemistry C on this work in August 2015 and is currently under review.
Current Progress
Design, Synthesis, and Characterization of Cu-containing MOFs
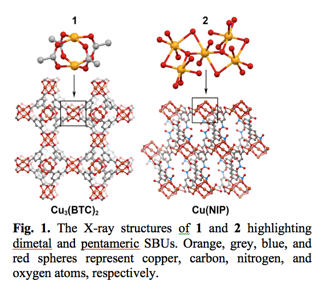
The initial criteria for the selection of MOFs to be studied by XPS were: high thermal stability, ability to retain the structure after MOF activation, and possibility to generate UMS. Additional restrictions, such as a difference in UMS ensemble sizes, were applied to the chosen MOFs to ensure an ability to explore the effect of UMS geometry. We also imposed the requirement that at least one of the chosen MOFs should possess a known behavior and be very well characterized by a variety of solid-state techniques. In this manner, we can establish clear structure-property relationships based on our experimental data and theoretical calculations in combination with previously reported studies. As a result, Cu3(BTC)2(H2O)3 (1, BTC = benzene-1,3,5-tricarboxylate) and [Cu5(NIP)4(OH)2(H2O)6]⋅(H2O)5 (2, NIP = 5-nitroisophthalate) were prepared by a solvothermal method (Fig. 1). Characterization of the synthesized MOFs were performed by X-ray crystallography, thermogravimetric analysis, and FT-IR spectroscopy.
As shown in Fig. 1,
1 contains dinuclear secondary building units (SBUs)
bridged by four carboxylate groups while 2
has an unusual pentameric copper-based SBU (Fig. 1).
 The activation
procedure developed for 1 and 2 facilitated removal of the solvent
molecules, which occupy the axial sites on each Cu2+ ion, and
resulted in formation of UMS. Based on our studies, 1 and 2 satisfy all
criteria mentioned above: they are thermally stable, able to preserve crystallinity after activation procedure, contain UMS, and
possess two distinctively different UMS geometries.
The activation
procedure developed for 1 and 2 facilitated removal of the solvent
molecules, which occupy the axial sites on each Cu2+ ion, and
resulted in formation of UMS. Based on our studies, 1 and 2 satisfy all
criteria mentioned above: they are thermally stable, able to preserve crystallinity after activation procedure, contain UMS, and
possess two distinctively different UMS geometries.
Investigation of Active Cu sites by XPS
For 1, XPS data for the Cu(2p3/2) region show two distinct peaks at 935.0 and 933.3 eV, which are assigned to Cu+2 and Cu+1, respectively (Fig. 2a). After heating the sample in the catalysis cell in flowing Ar for 14 hrs at 225 °C, there is a dramatic increase in the Cu+1/Cu+2 ratio from 0.76 to 5.6. This thermal treatment is believed to convert Cu+2 to Cu+1, given that more extensive heating in Ar (35 hrs) achieved ~100% complete conversion. Exposure of reduced 1 to O2 for 2 hrs at room temperature diminishes the Cu+1/Cu+2 ratio to a value of 1.5, suggesting that oxygen preferentially binds to Cu+1 sites and converts Cu+1 to Cu+2. Heating the O2-exposed sample to 275 °C in vacuum for 5 hrs does not change the peak shape, and this implies that desorption of oxygen does not occur.
The
observation of mixed copper oxidation states is also observed for 2 although the Cu+1/Cu+2
ratio is significantly higher for 1
(Fig. 2b). After heating 2
to 200 °C for 14 hrs in Ar, the Cu+1/Cu+2
ratio increased from 0.53 to 1.1. Upon exposure to O2 at room
temperature for 2 hrs, the Cu+1/Cu+2
ratio decreases to 0.76, suggesting oxidation of Cu+1 to Cu+2
and preferential binding of oxygen at Cu+1 sites. Heating 2 in vacuum for 2 hrs
at 200 °C increases the absolute
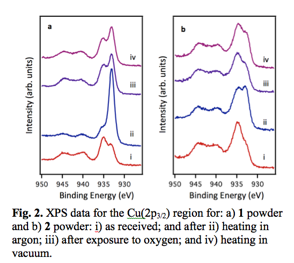 intensity of both peaks as adsorbates desorbed, but
the ratio of Cu+1/Cu+2 remains the same. Therefore, 1 and 2 exhibit similar behavior in the sense that mixed valences are
observed and thermal treatment enhances Cu+1 formation while
exposure to O2 oxidizes Cu+1 to Cu+2. However,
the Cu+2 in 1 is more
easily reduced than in 2 based on
the significantly higher Cu+1/Cu+2 ratio for 1; this could be attributed to the
differences in geometries and/or sizes of the UMS.
intensity of both peaks as adsorbates desorbed, but
the ratio of Cu+1/Cu+2 remains the same. Therefore, 1 and 2 exhibit similar behavior in the sense that mixed valences are
observed and thermal treatment enhances Cu+1 formation while
exposure to O2 oxidizes Cu+1 to Cu+2. However,
the Cu+2 in 1 is more
easily reduced than in 2 based on
the significantly higher Cu+1/Cu+2 ratio for 1; this could be attributed to the
differences in geometries and/or sizes of the UMS.
Ongoing Research
For the next phase of the project, the Shustova group is synthesizing bimetallic Cu-Zn MOFs with varying composition of the metals. The Chen group will investigate the binding of adsorbates (CO, O2, H2, methane) to the UMS in order to better understanding the nature of the active sites. In addition, we are working on the growth of MOF thin films that can be used for infrared spectroscopy studies of adsorbate binding.
Impact of Grant on PIs' Research Program
This grant has allowed the Chen and Shustova groups to combine their unique expertises in MOF synthesis and surface science to achieve detailed characterization of active sites for MOFs with different active site structures. Since these types of experiments have never been done before, this research is likely to lead to significant understanding that will be critical for the development of MOF catalysts. Furthermore, this work will have a major impact on future research directions in the Chen and Shustova groups, given that the current studies have defined new experimental approaches. Four female graduate students have participated in this project and had the opportunity to be involved in these new research directions.











