Reports: DNI554107-DNI5: Naphthenic Acid Ketonization Mechanism over Zeolites
Steven Crossley, University of Oklahoma
Carboxylic acids are abundant in petroleum derived streams in the form of naphthenic aicds, which commonly encounter acidic zeolites in petroleum refineries. If left unconverted, these acids can lead to corrosion and several other undesirable issues. Unfortunately, little is known about the mechanism of carboxylic acid transformation over acidic zeolites due to the complex series of reactions that succeeds the initial acid conversion. Our group has modified reaction conditons over the zeolite catalyst, H-ZSM5, to gain insight into this important reaciton. The initial transformation of carboxylic acids has been shown to occur through a ketonization reaction, where two organic acids are transformed to a ketone while removing oxygen in the form of water and CO2. Despite the fact that ketones have been observed as products from the tranformation of the simple probe molecule, acetic acid, over zeolites since the early 1980s, specific details of the reaction mechanism still remain elusive. While ketones have are reported in several cases, conditions that yield high selectivity to acetone while minimizing the sequential aldol condensation have yet to be identified. In addition to uncertainty surrounding the mechanism, the critical role of water, which is present in significant quantities in virtually all streams that contain acetic acid, on this reaction has not been investigated.
Identification of selective regime for ketonization
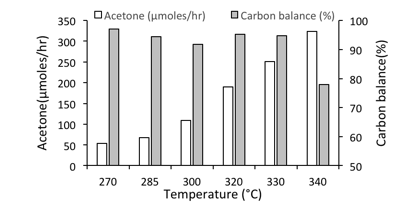
The ketonization over zeolites is surprisingly selective over a broad range of temperatures. Figure 1 shows the flow reaction of acetic acid on CBV8014 at various temperatures with W/F being constant in all the runs. These runs were performed at temperatures ranging from 270-340 C and as expected the yield of acetone increased with increasing temperature. High carbon balances were observed only up to a temperature of 330 C, beyond which a significant amount of carbon was lost due to heavier species that do not desorb from the catalyst. Though no condensation products were observed in our study, the drop in carbon balance beyond temperature of 330 C indicates their formation with higher temperatures required to desorb these species from the catalyst surface. The fact that most studies report a variety of subsequent aldol condensation products can clearly be mitigated by operating at milder temperatures under the conditions used in this study.
Figure 1:- Flow reaction studies of acetic acid on CBV8014 zeolite showing yields of acetone and carbon balance as a function of temperature.
Reaction mechanism
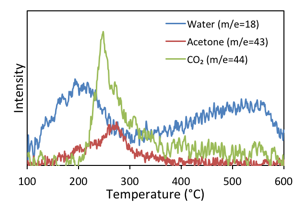
A temperature programmed desorption (TPD) experiment of acetic acid over CBV8014 was conducted to probe the reaction mechanism. Major desorbing species from the catalyst were water (m/e=18), CO2 (m/e=44), acetone (m/e=58) and m/e=43 (fragment of acetic acid and acetone). This profile shown in Figure 2 clearly indicates an initial dehydration step in the reaction pathway , likely resulting in the formation of an acylium intermediate. It is important to note that CO2 has a lower heat of adsorption on HZSM-5 as compared to that of water, so readsorption or bed effects could not explain the evolution of water prior to CO2. Quantification of the water produced during the dehydration step amounts to 16.75 μmoles, which corresponds to the dehydration of an acid on >82% of the 20.3 μmoles of Br¿nsted acid sites present in the catalyst bed.
Figure 2:- Acetic acid TPD on CBV8014 showing an initial dehydration step involved in the reaction pathway followed the decomposition of the intermediated to acetone and CO2.
FTIR analysis of the catalyst after exposure to acetic acid reveals the presence of acyl intermediates on the surface between the period of dehydration and coupling at ~150 C. These results indicate that the dehydration step is not the rate-determining step for the overall coupling reaction. Further insight may be obtained through kinetic fitting to determine the reaction order and deduce a rate-determining step.
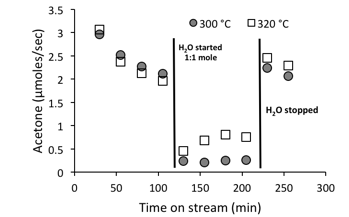
Numerous tests were conducted to ensure that the kinetic data were not limited by internal or external mass transfer. Under these conditions, we observe an apparent order of 1.5 with respect to acetic acid, which is due to a second order coupling rate determining step which is decreased by the influence of adsorbed species. An apparent order with respect to water of -0.3 is obtained, implying that water is competing for adsorption sites. Furthermore, the influence of water appears to hinder the rate of catalyst deactivation, so it has both positive and negative consequences on the reaction rate. This is illustrated by Figure 3, where the impact of water on the rate is less pronounced at higher temperatures, and a positive effect is observed in terms of catalyst deactivation rates.
Figure 3:- Acetic acid flow reactions at 300 C and 320 C showing a sudden drop in conversion when water was introduced.
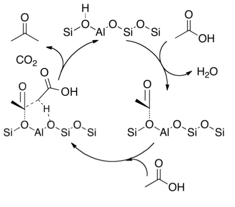
Based on this information, as well as some other experiments not included here, we have proposed the mechanism highlighted in Figure 4. More details regarding the nature of this coupling step, and how the second acid is activated and interacts with the zeolite are underway.
Figure 4:- Acetic acid TPD on CBV8014 showing an initial dehydration step involved in the reaction pathway followed the decomposition of the intermediated to acetone and CO2.
The critical importance of the acyl species on this reaction differentiates it from other solid acid catalyzed acid coupling reacitons presented in the literature. This also opens avenues for additional chemistry taking advantage of the surface intermediates that is a growing area of interest group's research that was made possible by these initial studies. Interesting cross ketonization studies are underway , highlighting the critical role of an alpha hydrogen within one of the species, and an acyl forming intermediate as the other. Cross coupling between benzoic acid, which does not contain an alpha hydrogen, with acetic or propionic acid, is observed, and carbon labeling experiments indicate that the benzoic acid is the acyl forming intermediate in cross coupling reactions, although it cannot couple with itself to form a ketone due to the lack of the alpha hydrogen.