Reports: ND754710-ND7: Graphenic Materials from Polycyclic Aromatic Hydrocarbon Oligomers
Mark C. Thies, Clemson University
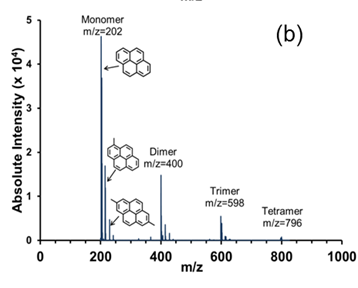
Thus, the goals of this work were to evaluate higher mol wt oligomers for mesogenic behavior – in particular, to isolate, structurally characterize, and analyze for mesophase pure trimer fractions from a pyrene pitch (see Figure 1) produced by the catalytic polymerization of pure pyrene monomer with AlCl3.
Figure 1. MALDI mass spectrum of a pyrene pitch produced catalytically using AlCl3.
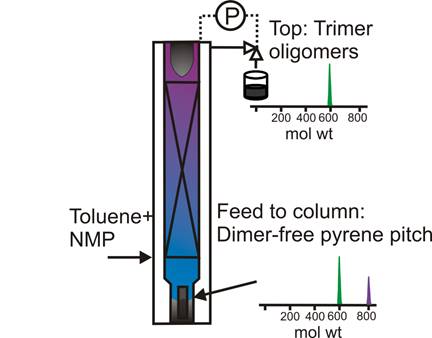
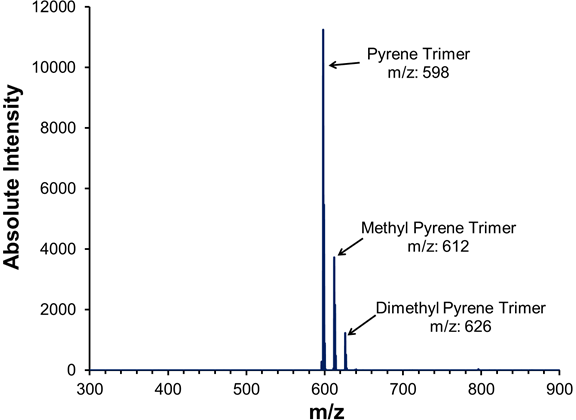
Preparation of a high-purity trimer fraction from catalytically produced pyrene pitch. A schematic of the semi-SCE process for producing pyrene trimer oligomers is given as Figure 2. The column operating pressure was set to a pressure of 79±2 bar, and a positive temperature gradient (+ΔT) of 330 °C (bottom), 350 °C (middle section) and 380 °C (top) was maintained across the column to enhance the purity of the top fractions. The detailed operation of this process for the fractionation of pyrene pitch is presented elsewhere (Esguerra et al., 2013). The composition of the fractions collected was determined by MALDI-MS. The MALDI mass spectrum of the pyrene trimer fraction, produced via semi-SCE with an NMP–toluene mixture as the extractive solvent, is given as Figure 3. Comparing these spectra to the MWDs of the starting materials in Figure 1, we see that a good separation of the trimers from the starting pitches was achieved. At first glance, the monodispersity of the pyrene pitch oligomers in Figure 1 is somewhat surprising, considering how highly polydisperse are other pitches produced catalytically from pure PAH components, such as naphthalene (Mochida et al., 2000). However, Sarofim and co-workers (1994) discovered that pyrene is highly resistant to fragmentation, with the products formed by polymerization and cyclodehydrogenation usually leaving the pyrene structure intact. Referring to the pyrene trimer spectrum in Figure 3, the minimal fragmentation that does occur during pyrene polymerization results in some methylation, consistent with the polymerization of PAHs such as naphthalene. MALDI intensities of methylated species are typically 5-10x higher compared to their actual concentration; thus, the pyrene trimer shown is ~95+% pure.
Figure 2. Semi-SCE apparatus used for producing pure trimer fractions from catalytically polymerized pyrene pitch.
Figure 3. MALDI mass spectrum of pyrene trimers produced catalytically with AlCl3 and isolated via semi-SCE, using a 15% NMP–toluene mixture as the extractive solvent.
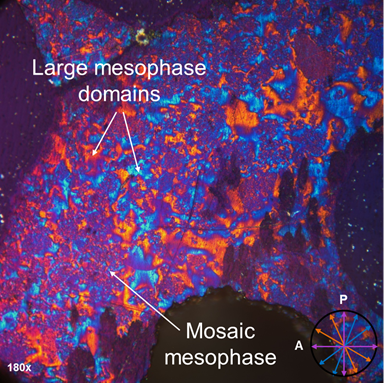
Mesophase analysis. Analysis of the pyrene trimer photomicrograph (Figure 4) reveals a fully developed liquid crystalline phase in which large mesophase domains combined with a fine mosaic texture are observed. This is first time that an unsubstituted PAH compound has ever been reported to form mesophase! (The degree of methyl substitution (<5%) is low enough to have had minimal impact on the system properties.) Furthermore, it is the lowest molecular weight PAH (i.e., 598 Da), whether a single compound or a mixture, for which mesophase has ever been observed. Finally, the softening point is also quite low, that is, 290 ºC.
Figure 4. Cross-polarized light photomicrograph of the pyrene trimer reveals a fully developed 100% mesophase. Mesophase layers oriented in the northeast–southwest direction (//) appear blue, in the northwest–southeast direction (\\) appear yellow-orange, and those oriented in the north–south and east—west directions appear magenta. Isotropic regions also appear magenta. P = polarizer; A = analyzer.
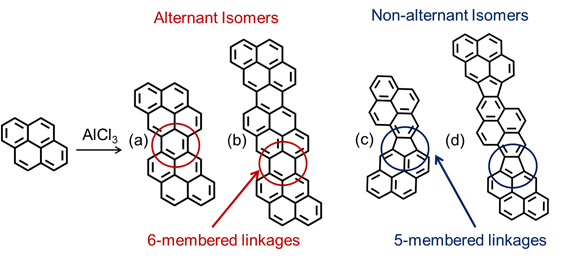
Representative structures for pyrene trimers in catalytically polymerized pyrene pitch are proposed in Figure 5 and are based on the previously identified alternant and non-alternant dimers of pyrene.
Conclusions. We have isolated the first unsubstituted, monodisperse PAH compound, namely a pyrene trimer, to form a fully developed liquid crystalline phase (i.e., 100% mesophase). Furthermore, with a molecular weight of 598 Da, this is the lowest mol wt PAH species for which the existence of mesophase has been reported. Although it is monodisperse, the pyrene trimer consists of multiple oligomers, as the monomer units are connected via either 5-membered (nonalternant) or 6-membered (alternant) rings in various configurations (even pyrene dimer has 5 isomers). Because of this isomeric character, pyrene trimer has a low melting point (290 °C), making the formation of a liquid crystalline phase possible. A pure PAH with the same molecular weight as our pyrene trimer fraction would have a solid–liquid melting point of 600 ºC or higher – and thus no region of liquid crystallinity.
Figure 5. Examples of possible molecular structures for the pyrene trimer isolated via SCE and shown in Figure 4. (a) Dinaphtho[2,1,8,7-defg:2’,1’,8’,7’-opqr]pentacene; (b) proposed alternant pyrene trimer; (c) Cyclopenta[1,2-a:3,4,5-c'd']dipyrene; (d) proposed non-alternant pyrene trimer.