Reports: DNI1052834-DNI10: Identification of Lithium Ion Battery Electrode Structural Inhomogeneity and Its Effect on Battery Performance
Likun Zhu, PhD, Indiana University-Purdue University, Indianapolis
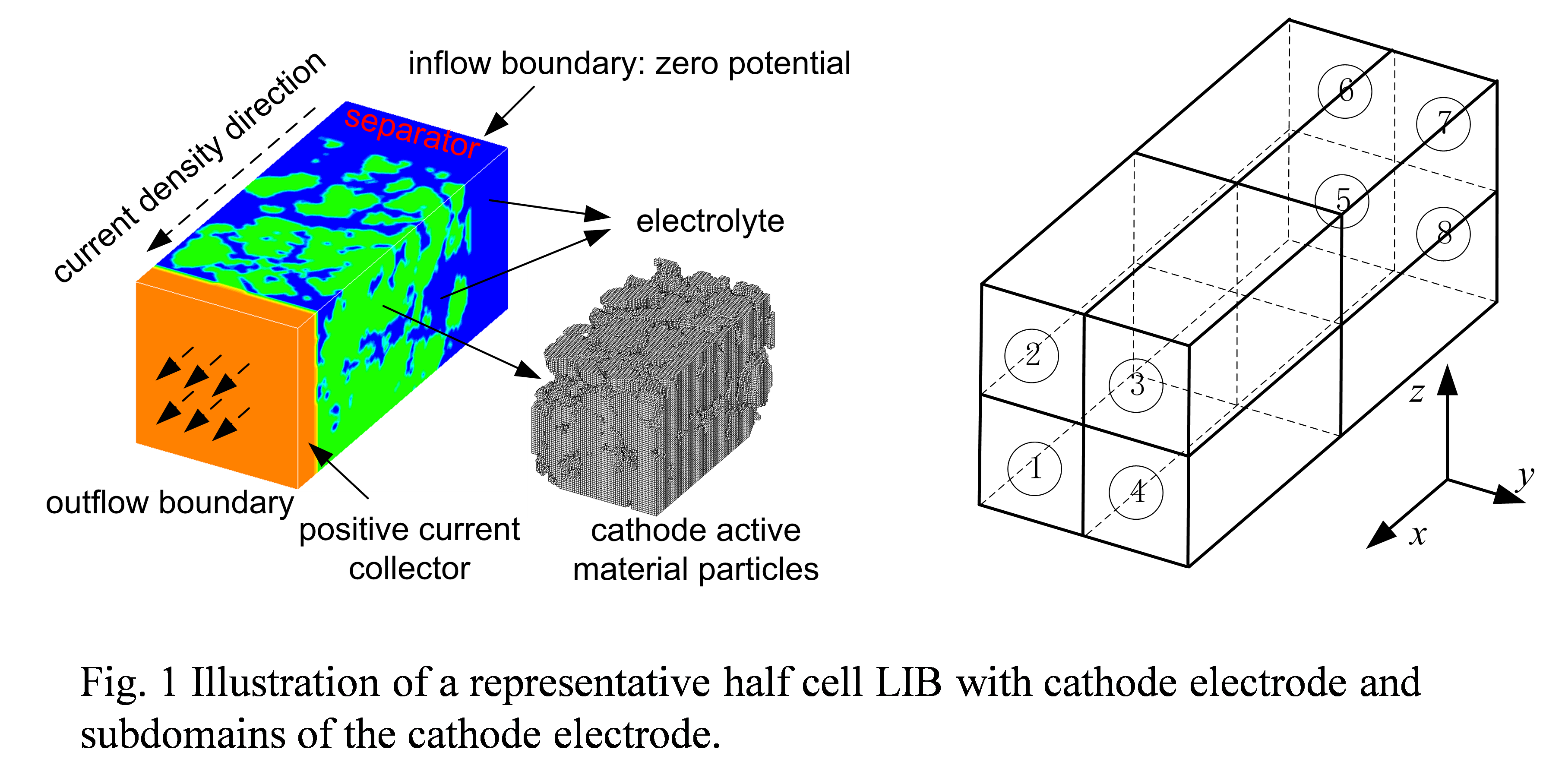
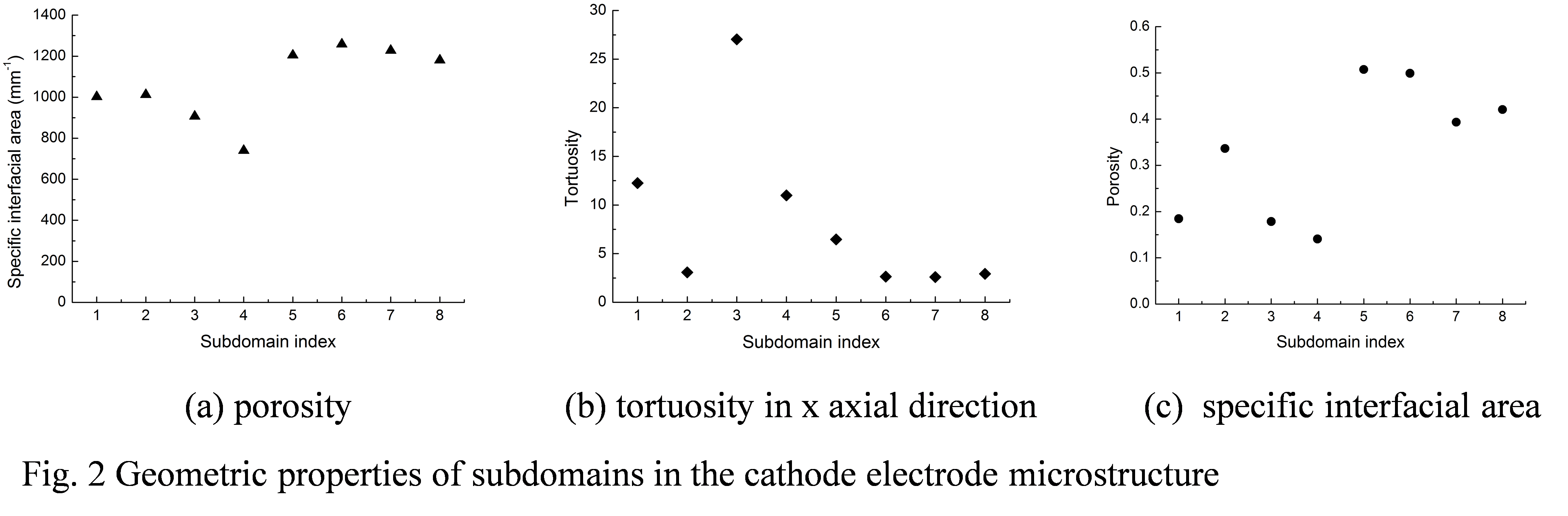
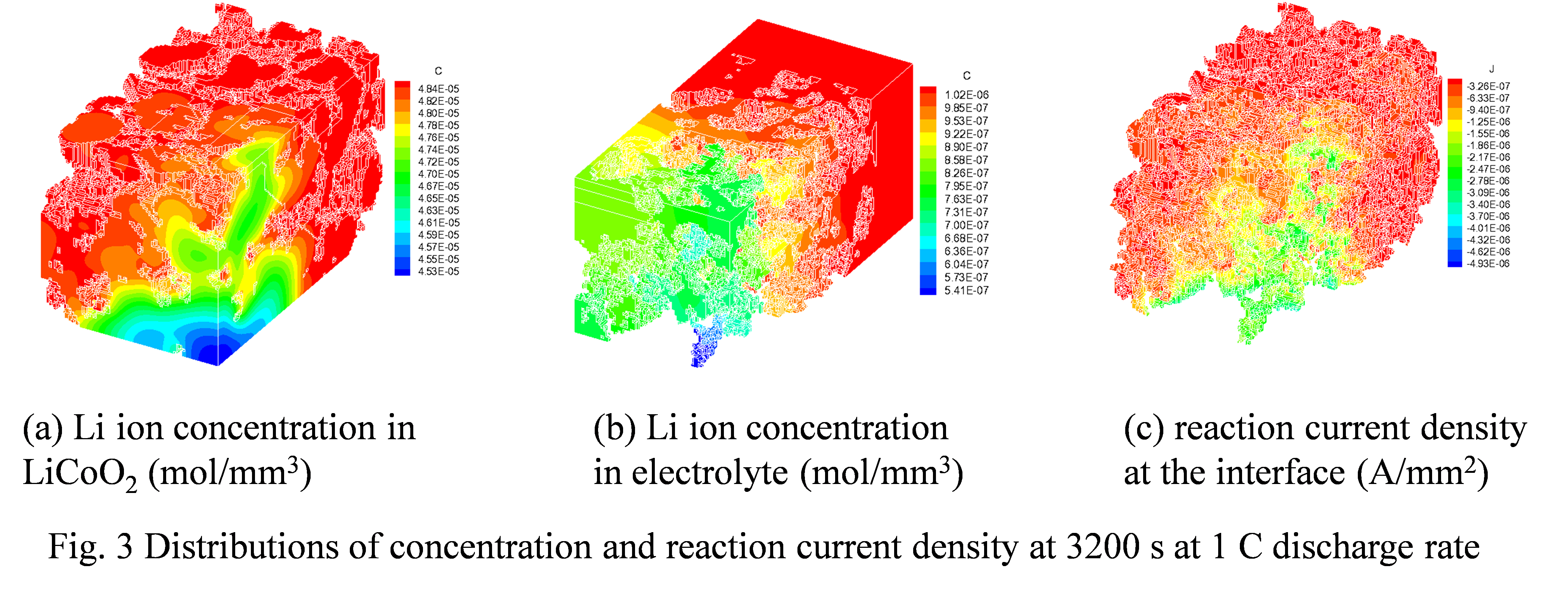
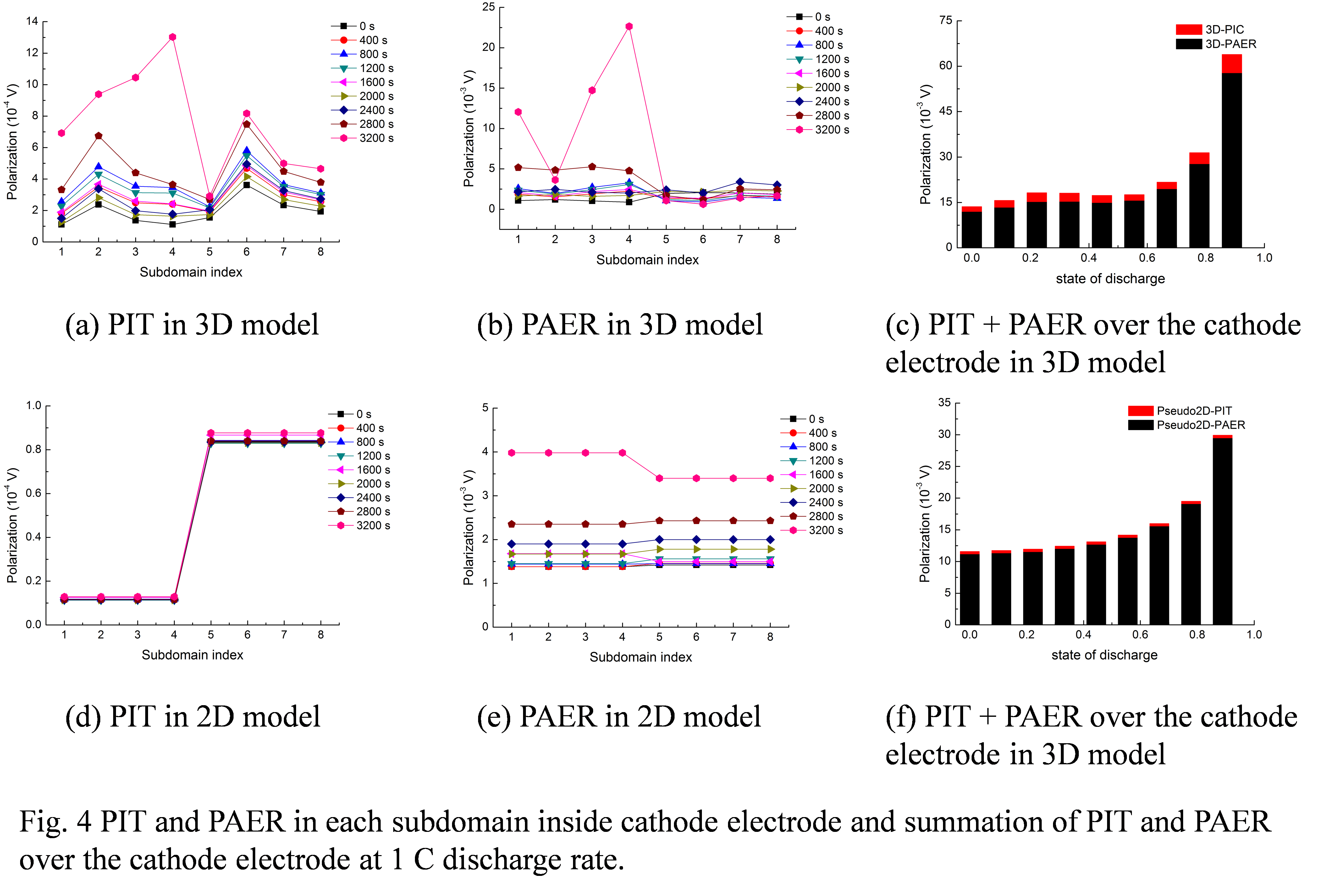
Likun Zhu, PhD, Indiana University-Purdue University, Indianapolis





Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society