Reports: UNI653258-UNI6: Spectroscopic Study of Graphene Halogenation Dynamics in the Gas Phase and in Solution: New Two-Dimensional Materials for Catalysis
Andrew C. Crowther, Ph.D., Barnard College
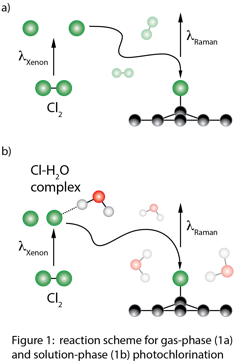
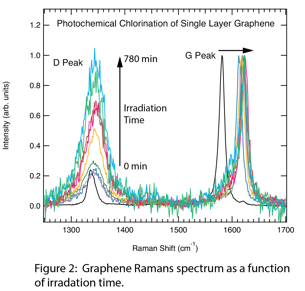
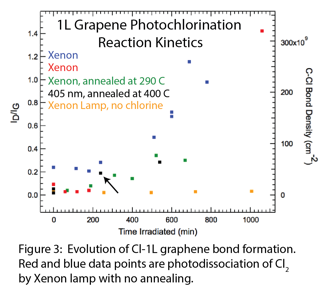

Andrew C. Crowther, Ph.D., Barnard College




Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society